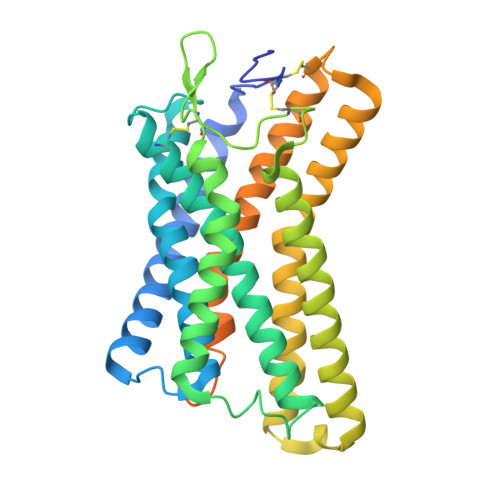
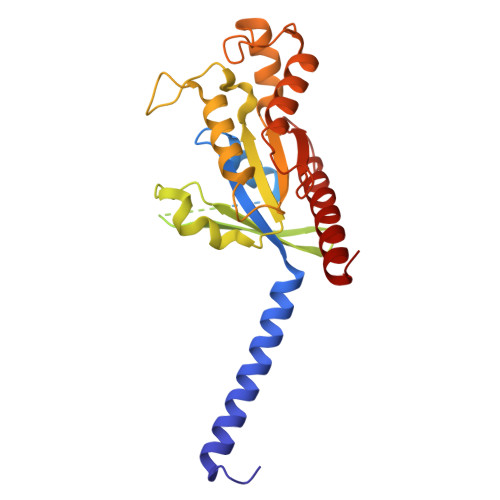
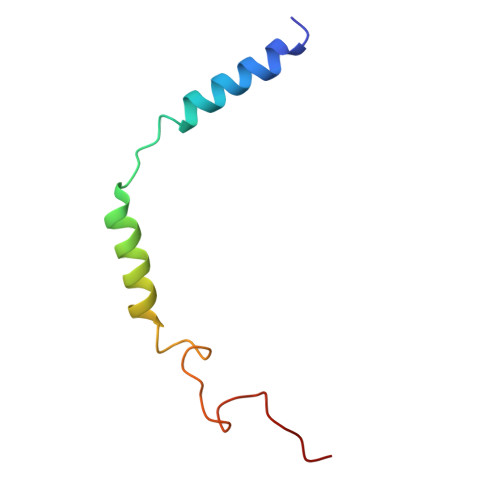
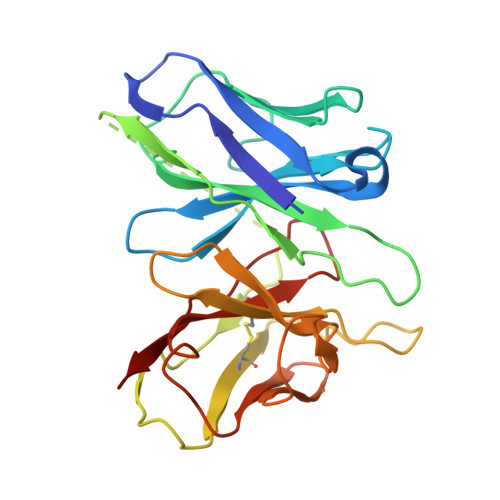
Ligand Recognition and Activation Mechanism of the Alicarboxylic Acid Receptors.
Liu, Y., Zhou, Z., Guan, F., Han, Z., Zhu, C., Ye, S., Yu, X., Qiao, A.(2024) J Mol Biology 436: 168795-168795
- PubMed: 39299383
- DOI: https://doi.org/10.1016/j.jmb.2024.168795
- Primary Citation of Related Structures:
9IQT - PubMed Abstract:
Endogenous ligands for alicarboxylic acid receptors are important metabolic intermediates that play a significant role in regulating body energy and maintaining homeostasis. However, the molecular mechanism of alicarboxylate ligand-mediated counterpart receptors is currently unclear. We resolve the active state structure of HCA2-niacin, and the structural analysis explains the mechanism of niacin selectivity in the alicarboxylic acid receptors family. Homology modeling, molecular dynamics simulation and mutagenesis experiments reveal different ligand recognition modes and activation mechanisms of the alicarboxylic acid receptors, analyze the flexibility of the binding pocket and elucidate the important role of disulfide bonds on receptor activation and ligand binding. These more detailed molecular mechanisms further elucidate the relevant mechanisms of human metabolism and provide key clues for subsequent drug development of alicarboxylic acid receptors.
- Tianjin Key Laboratory of Function and Application of Biological Macromolecular Structures, School of Life Sciences, Tianjin University, 92 Weijin Road, Nankai District, Tianjin 300072, China.
Organizational Affiliation: