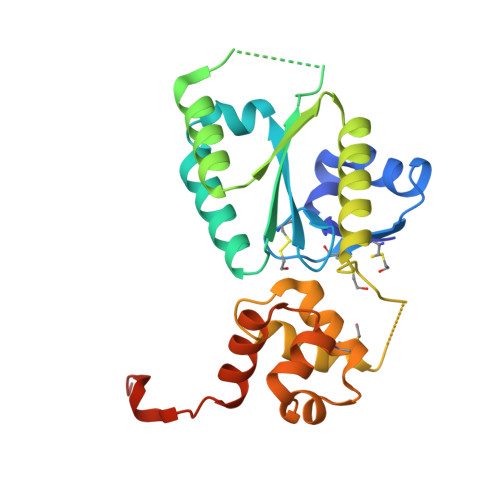
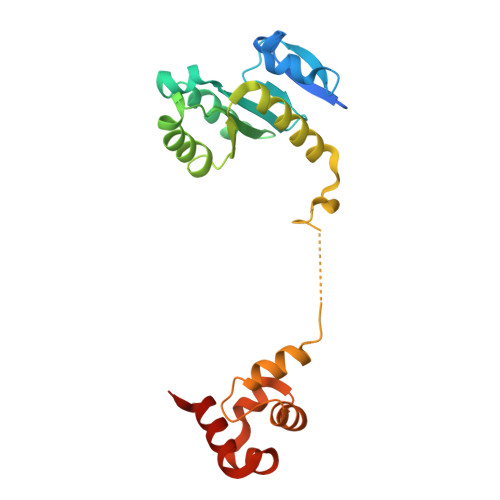
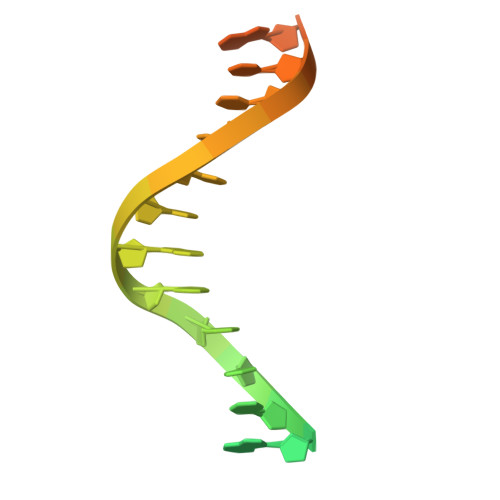
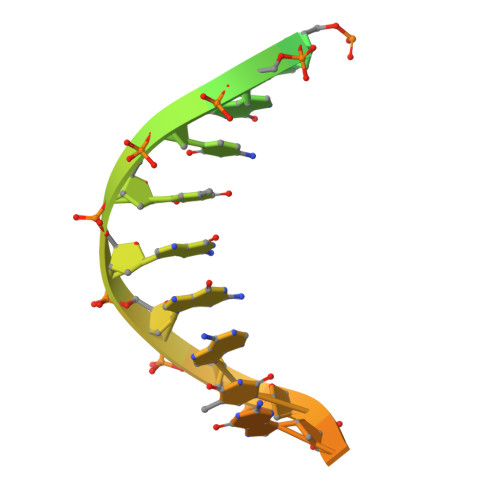
Structural basis of Fanconi anemia pathway activation by FANCM.
Bythell-Douglas, R., van Twest, S., Abbouche, L., Dunn, E., Coulthard, R.J., Briggs, D.C., Murphy, V., Zhang, X., Tan, W., Henrikus, S.S., Qian, D., Wu, Y., Wolf, J., Rigoreau, L., Shakeel, S., Chapman, K.L., McDonald, N.Q., Deans, A.J.(2025) EMBO J 44: 4013-4036
- PubMed: 40447800
- DOI: https://doi.org/10.1038/s44318-025-00468-3
- Primary Citation of Related Structures:
9EL5, 9HJO - PubMed Abstract:
FANCM is crucial in genome maintenance, functioning in the Fanconi anemia (FA) pathway, alternative lengthening of telomeres (ALT), and replication fork protection. FANCM recognizes branched DNA structures and promotes their remodeling through ATP-dependent branch migration. The protein has emerged as a promising therapeutic target due to synthetic lethal interactions with BRCA1, SMARCAL1, and RAD52, and in ALT-positive cancers. Here we present crystal structures of FANCM's N-terminal ATP-dependent translocase domain (2.2 Å) and C-terminal FAAP24-bound region (2.4 Å), both complexed with branched DNA. Through structural analysis, biochemical reconstitution, and cellular studies, we demonstrate that FANCM employs two distinct mechanisms: an ATP-dependent branch migration activity essential for DNA damage survival, and a branched DNA-binding mode that enhances FANCD2-FANCI monoubiquitination through FA core complex interaction. The N-terminal translocase domain specifically recognizes DNA junctions through multiple key elements, while the C-terminal FAAP24-binding domain engages adjacent double-stranded DNA. Our results reveal how FANCM evolved from an ancient DNA repair motor into a sophisticated sensor that couples DNA damage recognition to selective pathway activation, providing a structural framework for developing targeted therapeutics.
- Genome Stability Unit, St. Vincent's Institute of Medical Research, Fitzroy, VIC, Australia.
Organizational Affiliation: