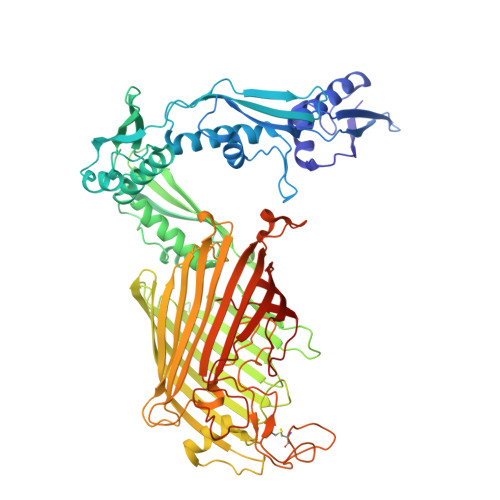
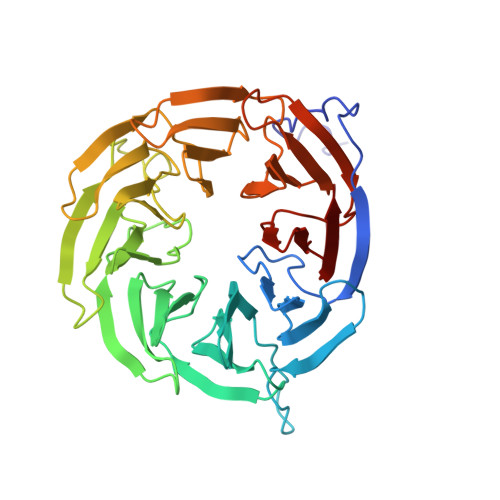
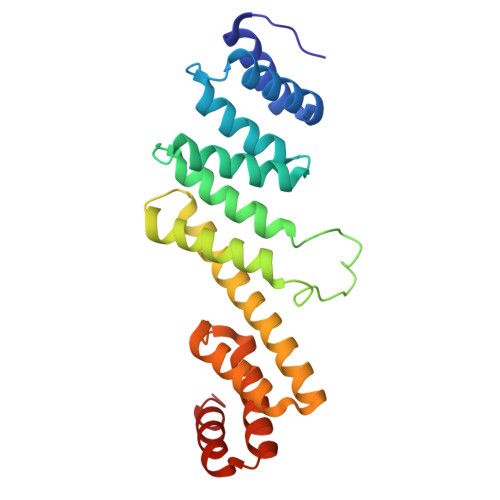
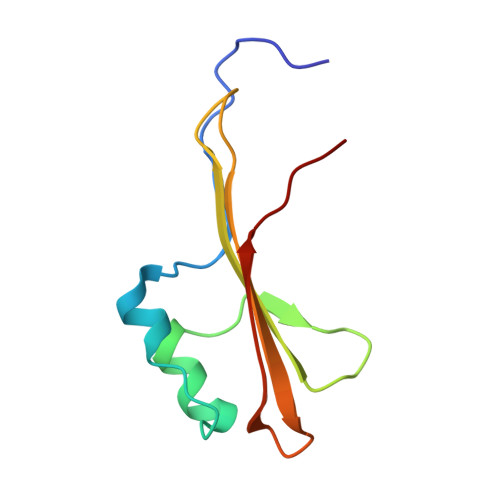
Molecular insights into how the motions of the beta-barrel and POTRA domains of BamA are coupled for efficient function.
Csoma, N., Machin, J.M., Whitehouse, J.M., Rodriguez-Alonso, R., Olejnik, M., Cahill, A.K., Cho, S.H., Schaberle, T.F., Iorga, B.I., Ranson, N.A., Radford, S.E., Calabrese, A.N., Collet, J.F.(2025) Nat Commun 16: 8832-8832
- PubMed: 41044071
- DOI: https://doi.org/10.1038/s41467-025-63897-y
- Primary Citation of Related Structures:
9H84, 9H85, 9H89 - PubMed Abstract:
The β-barrel assembly machinery (BAM) inserts β-barrel proteins into the outer membrane of Gram-negative bacteria, forming an essential permeability barrier. The core BAM component, BamA, is a β-barrel protein with an N-terminal periplasmic extension comprising five polypeptide transport-associated (POTRA) domains. Whilst BamA's structure is well characterised, it remains unclear how β-barrel and POTRA domain motions are coordinated. Using BamA variants with mutations in the hinge region between these two domains, we demonstrate that hinge flexibility is required for BAM function. Cryo-electron microscopy suggests that hinge rigidity impairs function by structurally decoupling these domains. A screen for spontaneous suppressors identified a mutation at position T434 in an extracellular loop of BamA, which has been previously shown to suppress BAM defects. Studying this variant provides insights into its function as a general rescue mechanism. Our findings underscore how BamA's sequence has been evolutionarily optimised for efficient function.
- WELBIO Department, WEL Research Institute, Wavre, Belgium.
Organizational Affiliation: