Scaffold-hopping, Bioisosterism and Structure-Based Design in the Development of Novel Acyl-ACP Thioesterase (Fat) Inhibitors as Potential Herbicides
Croft, R., Montgomery, M.G.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Oleoyl-acyl carrier protein thioesterase 1, chloroplastic | A [auth AaA] | 295 | Arabidopsis thaliana | Mutation(s): 0 Gene Names: FATA, FATA1, At3g25110, MJL12.5 EC: 3.1.2.14 | 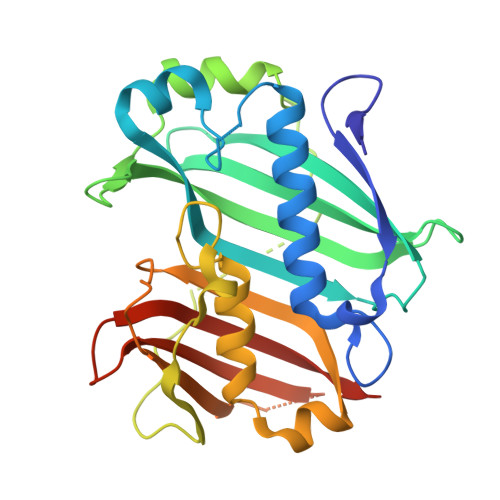 |
UniProt | |||||
Find proteins for Q42561 (Arabidopsis thaliana) Explore Q42561 Go to UniProtKB: Q42561 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q42561 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| A1IOW (Subject of Investigation/LOI) Query on A1IOW | B [auth AaA] | (1~{S},2~{R},4~{R})-1-methyl-2-[(2-methylphenyl)methoxy]-4-propan-2-yl-7-oxabicyclo[2.2.1]heptane C18 H26 O2 QMTNOLKHSWIQBE-UQJFVLDMSA-N |  | ||
| SO4 Query on SO4 | C [auth AaA], D [auth AaA] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | E [auth AaA] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 98.43 | α = 90 |
| b = 98.43 | β = 90 |
| c = 126.17 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| xia2 | data reduction |
| xia2 | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Other private | -- |