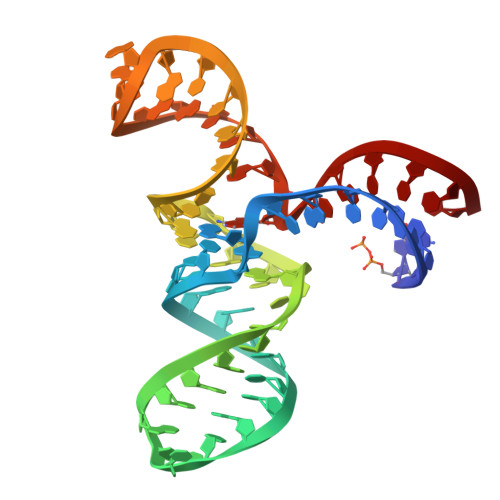
Structure and catalytic activity of the SAM-utilizing ribozyme SAMURI.
Chen, H.A., Okuda, T., Lenz, A.K., Scheitl, C.P.M., Schindelin, H., Hobartner, C.(2025) Nat Chem Biol
- PubMed: 39779902
- DOI: https://doi.org/10.1038/s41589-024-01808-w
- Primary Citation of Related Structures:
9FN2, 9FN3 - PubMed Abstract:
Ribozymes that catalyze site-specific RNA modification have recently gained increasing interest for their ability to mimic methyltransferase enzymes and for their application to install molecular tags. Recently, we reported SAMURI as a site-specific alkyltransferase ribozyme using S-adenosylmethionine (SAM) or a stabilized analog to transfer a methyl or propargyl group to N 3 of an adenosine. Here, we report the crystal structures of SAMURI in the postcatalytic state. The structures reveal a three-helix junction with the catalytic core folded into four stacked layers, harboring the cofactor and the modified nucleotide. Detailed structure-activity analyses explain the cofactor scope and the structural basis for site selectivity. A structural comparison of SAMURI with SAM riboswitches sheds light on how the synthetic ribozyme overcomes the strategies of natural riboswitches to avoid self-methylation. Our results suggest that SAM and its analogs may serve as substrates for various RNA-catalyzed reactions, for which the corresponding ribozymes remain to be identified.
- Institute of Organic Chemistry, Julius-Maximilians-Universität Würzburg, Würzburg, Germany.
Organizational Affiliation: