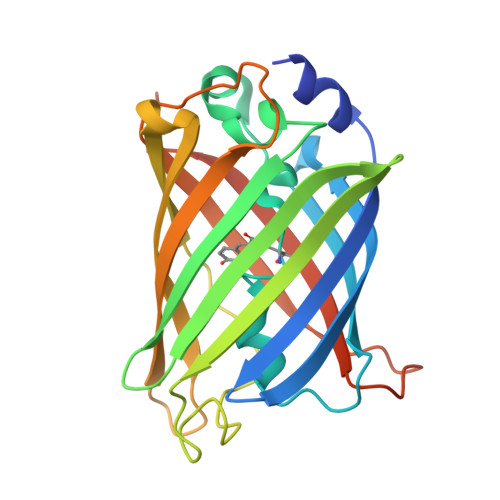
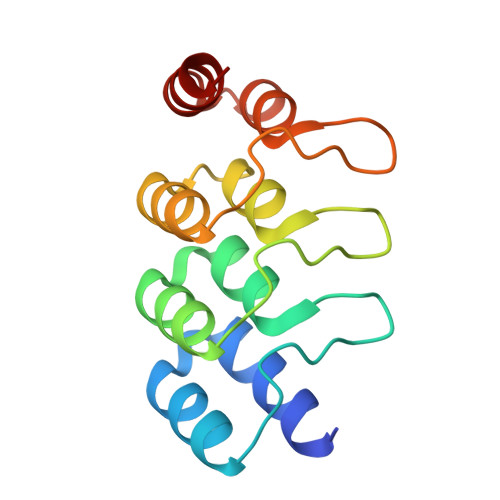
Molecular features defining the efficiency of bioPROTACs.
Winkelvoss, D., Vukovic, D., Hanny, A.C., Riermeier, L., Udovcic, A., Kolibius, J., Honegger, A., Mittl, P.R.E., Michel, E., Hansen, S., Pluckthun, A.(2025) Commun Biol 8: 946-946
- PubMed: 40542219
- DOI: https://doi.org/10.1038/s42003-025-08352-w
- Primary Citation of Related Structures:
9F22, 9F23, 9F24 - PubMed Abstract:
BioPROTACs consist of a target-binding unit and a component of the ubiquitin-proteasome system. However, the specific biophysical features influencing their effectiveness are poorly understood. We investigated the design principles defining the target-binding moiety of bioPROTACs. We used our recently developed assay for accurately measuring degradation rates, based on microinjection and live-cell microscopy, independent of other confounding factors like biosynthesis and transport. We used a very efficient UPS interaction domain from CHIP E3 ligase, and 9 different well-characterized DARPins to test degradation of the proof-of-principle target eGFP. All but two DARPins work well in this context, one sterically preventing E2 binding in the complex, the other overlapping with the target ubiquitination epitope. Affinity and thermodynamic stability of the binders had only a modest role. BioPROTACs constructed in this way were able to degrade eGFP catalytically. DARPins by themselves could also accelerate degradation of bound GFP, using other cellular E3 systems, but in a non-catalytic manner. The most important factor for efficient degradation by a bioPROTAC in trans is the correct orientation of the complex for target ubiquitination and presentation to the proteasome, still to be determined empirically. The strategies developed here show an efficient pathway to characterize and optimize such systems.
- Department of Biochemistry, University of Zurich, Zurich, Switzerland.
Organizational Affiliation: