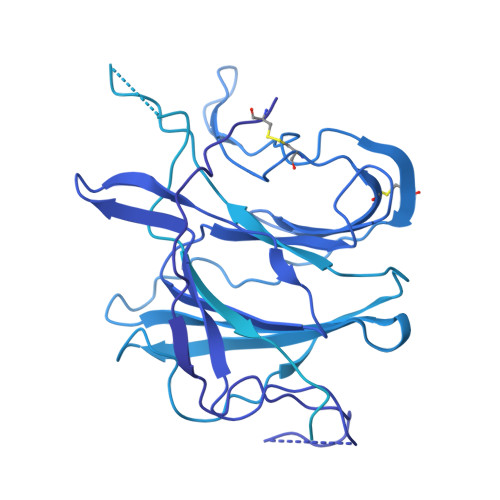
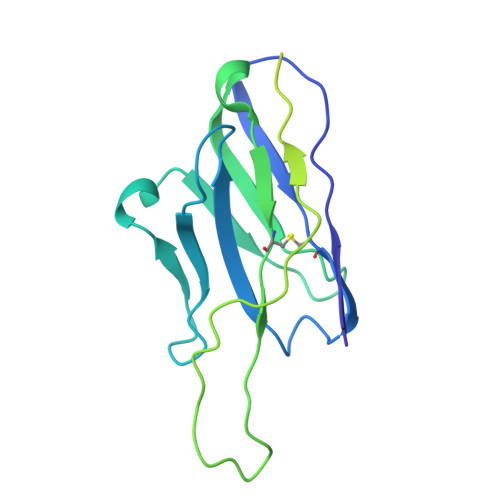
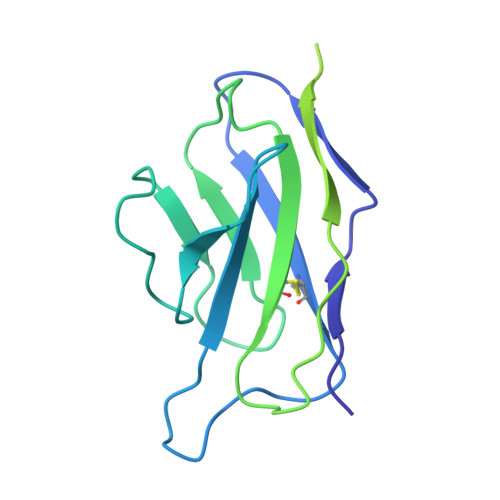
First-generation N-terminal domain supersite public antibodies retain activity against Omicron-derived lineages and protect mice against Omicron BA.5 challenge.
Dussupt, V., Jensen, J.L., Thomas, P.V., Mendez-Rivera, L., Lal, K.G., Zemil McCrea, M., Swafford, I., Hernandez, J., Sankhala, R.S., Rao, M., Arora, J., Hajduczki, A., Jian, N., Rees, P.A., Showell-De Leon, I., Smith, G., Smith, L., Wasson, D., Schmid, A., Teng, I.T., Zhou, T., Kwong, P.D., Currier, J.R., Reiley, W.W., Paquin-Proulx, D., Polonis, V.R., Collins, N.D., Michael, N.L., Joyce, M.G., Krebs, S.J.(2025) mBio 16: e0103625-e0103625
- PubMed: 40882161
- DOI: https://doi.org/10.1128/mbio.01036-25
- Primary Citation of Related Structures:
9ECX, 9ECY, 9ECZ, 9MI3 - PubMed Abstract:
Monoclonal antibodies to SARS-CoV-2 can offer prophylactic and therapeutic protection against severe disease, with particular utility for immunosuppressed and vulnerable populations. With the constant emergence of new variants, understanding the neutralizing potency of monoclonal antibodies to dynamic spike protein epitopes is crucial. We show that a set of VH1-24-derived N-terminal domain (NTD)-directed antibodies, isolated from a convalescent donor early in the pandemic, displayed remarkable neutralization resilience against many Omicron SARS-CoV-2 variants, including BA.2, BA.5, and BQ.1.1. Neutralization potency to these Omicron variants is associated with slower off-rates to the spike protein. Structural characterization of the most potent NTD antibody, WRAIR-2008, revealed a conserved mode of interaction shared with other antibodies of the same multi-donor class. WRAIR-2008 protected mice from weight loss following BA.5 challenge and reduced infectious viral titers in the lungs. Our study highlights the retention of neutralization activity and protection of first-generation VH1-24-derived NTD-directed antibodies to specific Omicron variants and provides valuable insights into the shifting landscape of SARS-CoV-2 variants that are vulnerable to select monoclonal antibodies. As SARS-CoV-2 circulating variants evolve, it is important to understand the vulnerabilities of these viruses to neutralizing antibodies. Within this manuscript, we describe first-generation antibodies isolated following infection with WA-1 that retain viral neutralization to subsequent Omicron variants by targeting a site of viral vulnerability called the NTD. This work highlights the shifting landscape of SARS-CoV-2 variants and provides mechanistic insights into how antibodies from prior infections may play a role in preventing subsequent SARS-CoV-2 variant infections.
- Viral Diseases Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA.
Organizational Affiliation: