Diverse modulation mechanisms of human Nav1.6 by gating modifier neurotoxins
Fan, X., Jian, H., Chen, J., Wang, H., Wu, Q., Liu, T., Guo, Q., Shen, Z., Pan, X., Jin, X., Yan, N.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Sodium channel protein type 8 subunit alpha | 1,980 | Homo sapiens | Mutation(s): 0 Gene Names: SCN8A, MED | 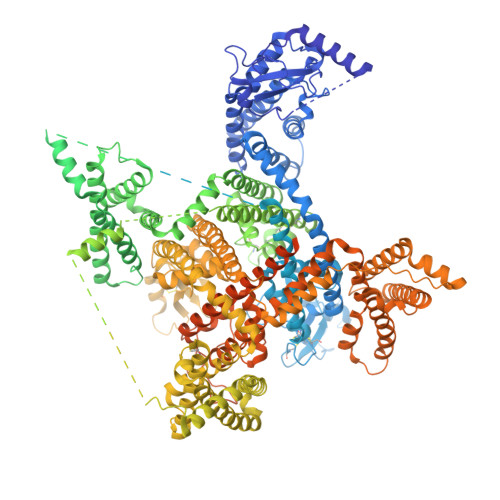 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9UQD0 (Homo sapiens) Explore Q9UQD0 Go to UniProtKB: Q9UQD0 | |||||
PHAROS: Q9UQD0 GTEx: ENSG00000196876 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9UQD0 | ||||
Glycosylation | |||||
| Glycosylation Sites: 5 | Go to GlyGen: Q9UQD0-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Sodium channel subunit beta-1 | B [auth C] | 218 | Homo sapiens | Mutation(s): 0 Gene Names: SCN1B | 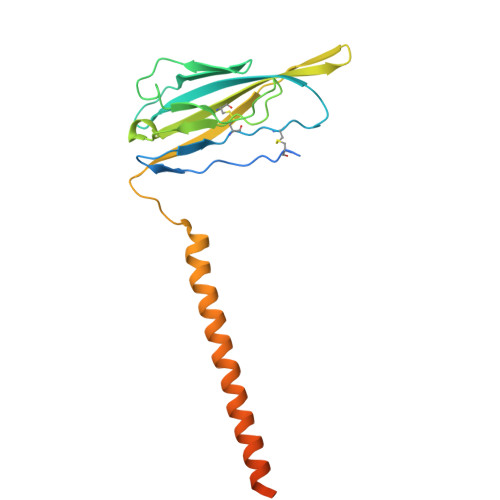 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q07699 (Homo sapiens) Explore Q07699 Go to UniProtKB: Q07699 | |||||
PHAROS: Q07699 GTEx: ENSG00000105711 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q07699 | ||||
Glycosylation | |||||
| Glycosylation Sites: 4 | Go to GlyGen: Q07699-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Beta-theraphotoxin-Ps1a | C [auth H], D [auth I], E [auth J] | 34 | Paraphysa scrofa | Mutation(s): 0 | 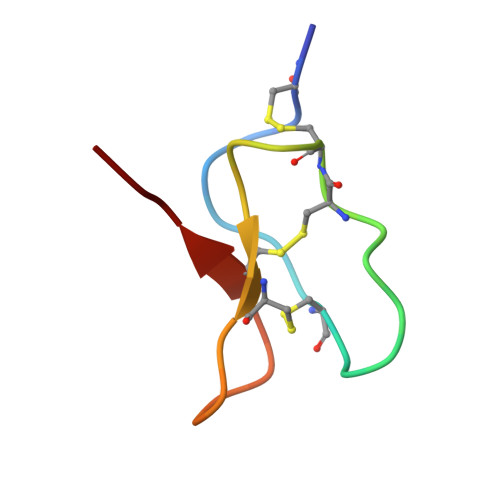 |
UniProt | |||||
Find proteins for P84510 (Paraphysa scrofa) Explore P84510 Go to UniProtKB: P84510 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P84510 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-6)-[alpha-D-mannopyranose-(1-3)]alpha-D-mannopyranose-(1-6)-[alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | F [auth D] | 9 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G83161QT GlyCosmos: G83161QT GlyGen: G83161QT | |||||
| Ligands 8 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| P5S Query on P5S | S [auth A] | O-[(R)-{[(2R)-2,3-bis(octadecanoyloxy)propyl]oxy}(hydroxy)phosphoryl]-L-serine C42 H82 N O10 P TZCPCKNHXULUIY-RGULYWFUSA-N |  | ||
| PCW Query on PCW | M [auth A], O [auth A], Q [auth A], R [auth A] | 1,2-DIOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE C44 H85 N O8 P SNKAWJBJQDLSFF-NVKMUCNASA-O |  | ||
| P3X Query on P3X | W [auth A] | (5E,17R,20S)-23-amino-20-hydroxy-14,20-dioxo-15,19,21-trioxa-20lambda~5~-phosphatricos-5-en-17-yl hexadecanoate C35 H68 N O8 P IXIBEFBSXYIWMP-VCRBCDDQSA-N |  | ||
| 9Z9 Query on 9Z9 | V [auth A] | (3beta,14beta,17beta,25R)-3-[4-methoxy-3-(methoxymethyl)butoxy]spirost-5-en C34 H56 O5 CEEBZAXXSRFQIC-GZSGZGDASA-N |  | ||
| LPE Query on LPE | T [auth A] | 1-O-OCTADECYL-SN-GLYCERO-3-PHOSPHOCHOLINE C26 H57 N O6 P XKBJVQHMEXMFDZ-AREMUKBSSA-O |  | ||
| Y01 Query on Y01 | L [auth A], P [auth A], U [auth A] | CHOLESTEROL HEMISUCCINATE C31 H50 O4 WLNARFZDISHUGS-MIXBDBMTSA-N |  | ||
| CLR Query on CLR | N [auth A] | CHOLESTEROL C27 H46 O HVYWMOMLDIMFJA-DPAQBDIFSA-N |  | ||
| NAG Query on NAG | H [auth A] I [auth A] J [auth A] K [auth A] X [auth C] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| Funding Organization | Location | Grant Number |
|---|---|---|
| Human Frontier Science Program (HFSP) | France | LT000754/2020-L |