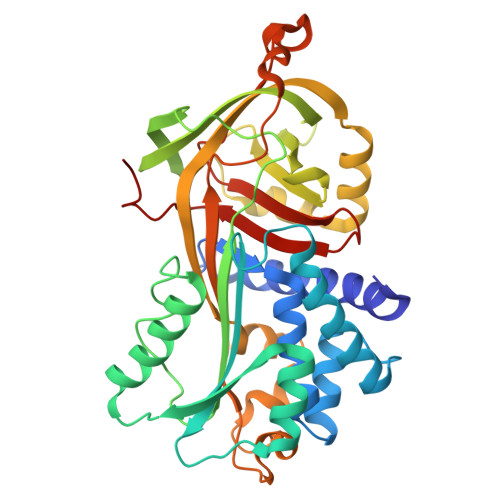
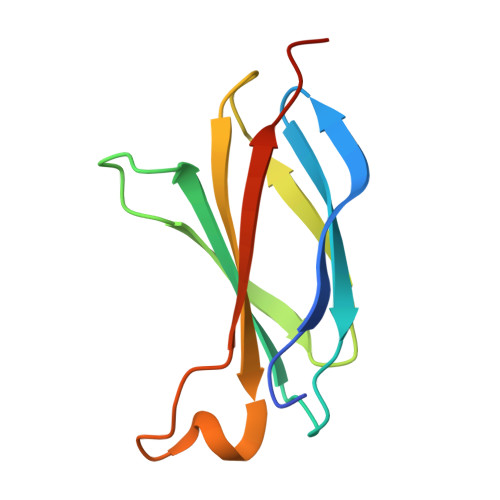
Improving the diffraction quality of heat-shock protein 47 crystals.
Kish, K., Cobell, S., Szapiel, N., Yan, C., Newitt, J.A., Tredup, J., Rodrigo, I., Tomasco, E., Gao, M., Marsilio, F., Haugner, J., Lipovsek, D., Deng, B., Bousquet, P., Zhang, Y., Schmidt, H., Sheriff, S.(2024) Acta Crystallogr F Struct Biol Commun 80: 302-313
- PubMed: 39397789
- DOI: https://doi.org/10.1107/S2053230X24009233
- Primary Citation of Related Structures:
9CQE, 9CQF, 9CQG, 9CQH, 9CQI, 9CQJ - PubMed Abstract:
Heat-shock protein 47 (HSP47) is a potential target for inhibitors that ameliorate fibrosis by reducing collagen assembly. In an effort to develop a structure-based drug-design system, it was not possible to replicate a previous literature result (PDB entry 4au4) for apo dog HSP47; instead, crystal forms were obtained in which pairs of dog HSP47 molecules interacted through a noncleavable C-terminal His-tag to build up tetramers, all of which had multiple molecules of HSP47 in the asymmetric unit and none of which diffracted as well as the literature precedent. To overcome these difficulties, a two-pronged approach was followed: (i) the His-tag was moved from the C-terminus to the N-terminus and was made cleavable, and (ii) Adnectin (derived from the tenth domain of human fibronectin type III) crystallization chaperones were developed. Both approaches provided well diffracting crystals, but the latter approach yielded crystal forms with only one or two HSP47 complexes per asymmetric unit, which made model building less onerous.
- Molecular Structure & Design, Bristol Myers Squibb Research & Development, PO Box 4000, Princeton, NJ 08543-4000, USA.
Organizational Affiliation: