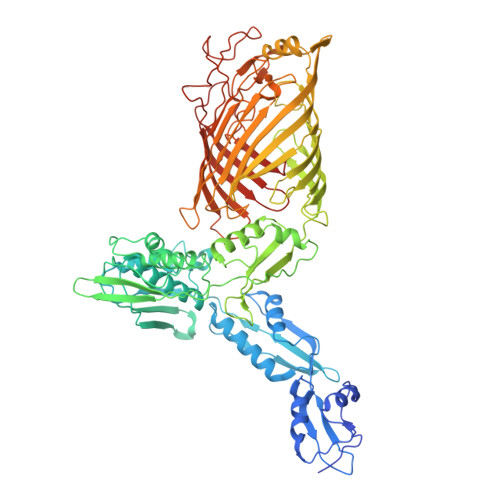
Structural insights into outer membrane protein biogenesis in pathogenic Neisseria.
Billings, E., Fan, Z., Sooreshjani, M.A., Gumbart, J.C., Noinaj, N.(2025) Structure 33: 1893
- PubMed: 40902586
- DOI: https://doi.org/10.1016/j.str.2025.08.009
- Primary Citation of Related Structures:
9CMW, 9CN0, 9CN1 - PubMed Abstract:
N. gonorrhoeae (Ngo) causes the sexually transmitted infection gonorrhea with ∼106 million infections worldwide annually. Ngo infections can result in an increased risk of acquiring HIV, infertility, and blindness. To combat Ngo infections, we report the cryoelectron microscopy (cryo-EM) structure of the Ngo β-barrel assembly machinery (NgBAM), which is responsible for the biogenesis of β-barrel outer membrane proteins (OMPs). NgBAM was observed in an inward-open state; however, the polypeptide transport-associated (POTRA) domains more closely match those found in the outward-open state in E. coli β-barrel assembly machinery (BAM). The barrel seam of NgBamA consists of partial pairing of strand β1 with β16; no outward-open state of NgBAM was observed. Molecular dynamics (MD) simulations reveal unique overall dynamics and interplay between the POTRA domains of NgBamA and NgBamD. We propose that in Ngo, initial recognition occurs in the inward-open state where the last strand of the OMP partially pairs with β1 of NgBamA and must compete off β16.
- Markey Center for Structural Biology, Department of Biological Sciences, Purdue University, West Lafayette, IN 47907, USA.
Organizational Affiliation: