Ran modulates allosteric crosstalk between importin beta surfaces.
Ko, Y.H., Li, F., Suinn, S.S., Li, J., Hou, C.D., Lokareddy, R.K., Cingolani, G.(2025) Nat Commun 16: 11425-11425
- PubMed: 41381450
- DOI: https://doi.org/10.1038/s41467-025-66255-0
- Primary Citation of Related Structures:
9BFC - PubMed Abstract:
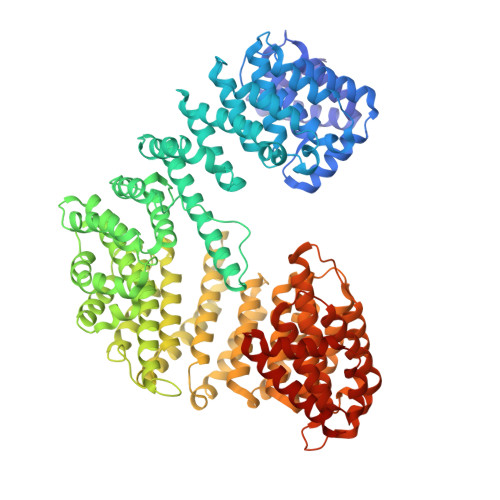
A cellular gradient of the GTPase Ran orchestrates the movement of import and export complexes through the Nuclear Pore Complex (NPC). Ran-GTP modulates two essential activities of importin β during nuclear import. On the one hand, it reduces the avidity of importin β for phenylalanine-glycine-rich nucleoporins (FG-nups), thereby facilitating the passage of import complexes through the permeability barrier. On the other hand, it disassembles import complexes, releasing the import cargo into the nucleus. The precise mechanisms by which Ran-GTP modulates importin β activities have remained hypothetical. Leveraging cryogenic electron microscopy (cryo-EM) single-particle analysis, in this paper, we describe five distinct conformational states of importin β in complex with various effectors encountered during an import reaction, specifically IBB-cargos, FG-repeats, Ran-GTP, Ran-GTP:RanBP1, and Ran-GDP:RanBP1. Comparing these states allows us to decipher the conformational landscape of importin β without interference from crystallization agents and lattice forces. By correlating structural data with biochemical activities, we find that Ran-GTP, but not Ran-GDP, constrains the solenoid structure of importin β, closing high-affinity FG-binding pockets and displacing import cargos through allosteric crosstalk between the concave and convex surfaces. We propose that this allosteric mechanism is relevant to other β-karyopherins involved in nuclear import.
- Department of Biochemistry and Molecular Genetics, The University of Alabama at Birmingham, Birmingham, AL, USA.
Organizational Affiliation: