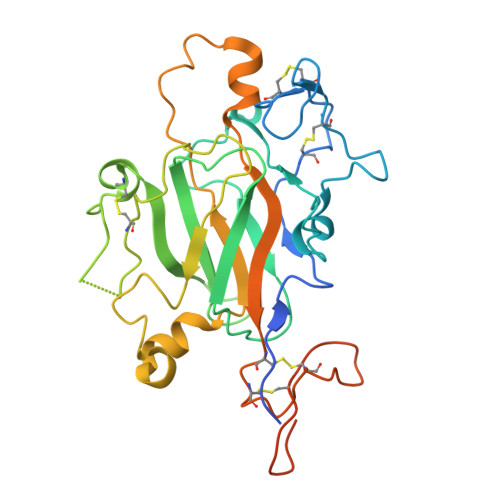
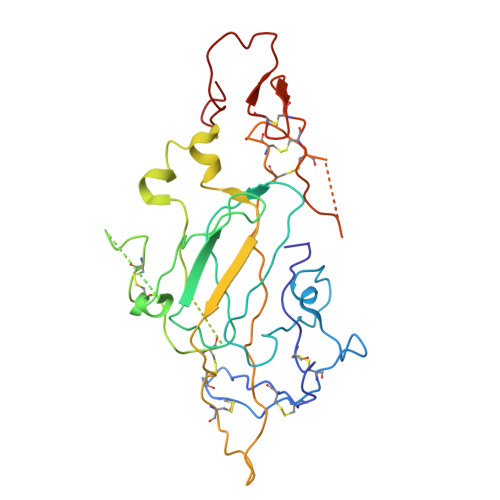
Cryo-EM reveals molecular mechanisms underlying the inhibitory effect of netrin-4 on laminin matrix formation.
Kulczyk, A.W., McKee, K.K., Yurchenco, P.D.(2025) Nat Commun 16: 7256-7256
- PubMed: 40769979
- DOI: https://doi.org/10.1038/s41467-025-62814-7
- Primary Citation of Related Structures:
9AZ3 - PubMed Abstract:
Netrin-4 is a tumor suppressor that interferes with formation of the laminin lattice. We employed cryo-electron microscopy to determine a structure of the protein complex consisting of the N-terminal fragments from netrin-4 and laminin γ1. The structure reveals that netrin-4 binds laminin γ1 at the molecular interface where laminin β1 would have bound, thus inhibiting the assembly of the heterotrimeric laminin polymer nodes consisting of α1, β1, and γ1 subunits, and their polymerization into the extracellular lattice. The four orders of magnitude higher affinity of the netrin-4-laminin γ1 interaction results from the larger buried surface area than the one formed by β1 and γ1 laminins and greater electrostatic surface complementarity. Our findings, supported by site-directed mutagenesis, solid-phase binding analysis, laminin polymerization, and Schwann cell assays, collectively demonstrate that, in addition to inhibiting laminin polymerization, netrin-4 disassembles the pre-existing laminin lattice. The structure has the potential to facilitate the development of novel therapies for cancer treatment.
- Institute for Quantitative Biomedicine, Department of Biochemistry and Microbiology, Rutgers University, Piscataway, NJ, USA. arek.kulczyk@rutgers.edu.
Organizational Affiliation: