BL03HB: a Laue microdiffraction beamline for both protein crystallography and materials science at SSRF
Wang, Z.J., Wang, S.S., Su, Z.H., Yu, L., Wang, Y.Z., Sun, B., Wen, W., Gao, X.Y.(2024) Nucl Sci Tech 35: 119
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2024) Nucl Sci Tech 35: 119
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Lysozyme C | 129 | Asplenium bulbiferum subsp. bulbiferum | Mutation(s): 0 EC: 3.2.1.17 | 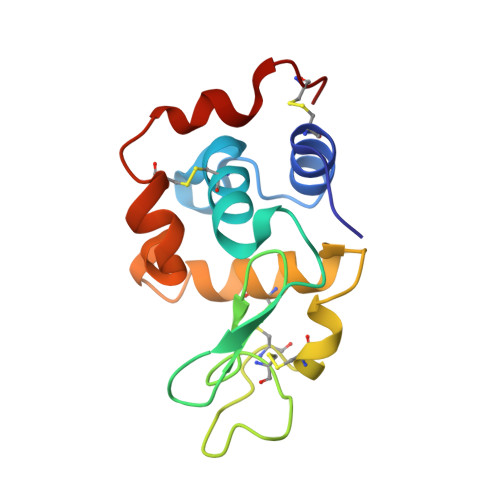 | |
UniProt | |||||
Find proteins for P00698 (Gallus gallus) Explore P00698 Go to UniProtKB: P00698 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00698 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 78.56 | α = 90 |
| b = 78.56 | β = 90 |
| c = 37.5 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| LaueView | data reduction |
| pointless | data scaling |
| PHASES | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Basic Research Program of China (973 Program) | China | -- |