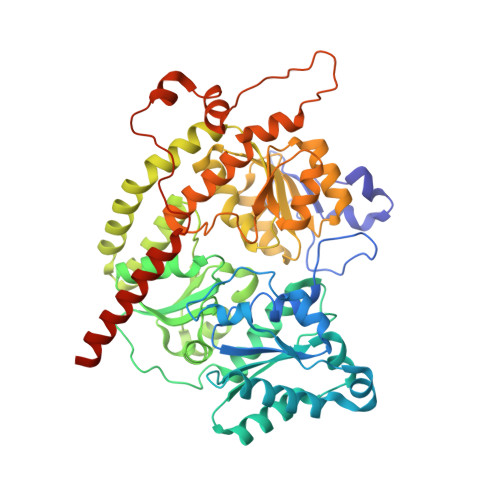
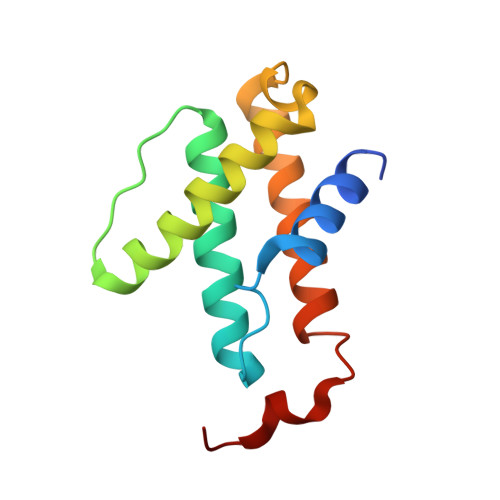
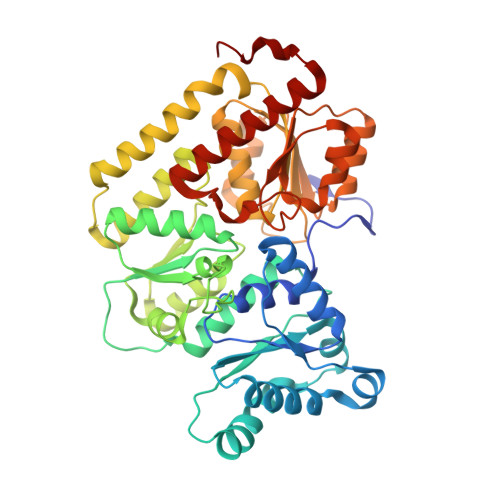
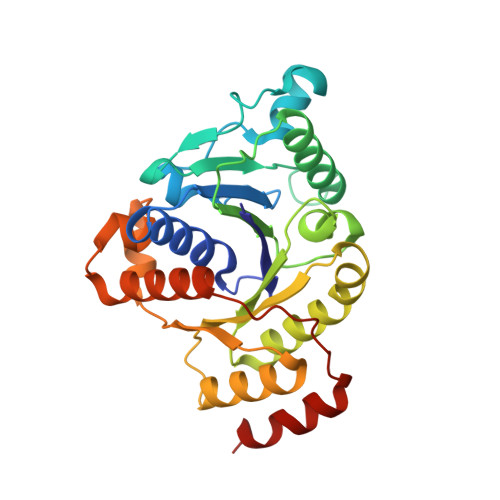
Structural insights into the iron nitrogenase complex.
Schmidt, F.V., Schulz, L., Zarzycki, J., Prinz, S., Oehlmann, N.N., Erb, T.J., Rebelein, J.G.(2024) Nat Struct Mol Biol 31: 150-158
- PubMed: 38062208
- DOI: https://doi.org/10.1038/s41594-023-01124-2
- Primary Citation of Related Structures:
8OIE, 8PBB - PubMed Abstract:
Nitrogenases are best known for catalyzing the reduction of dinitrogen to ammonia at a complex metallic cofactor. Recently, nitrogenases were shown to reduce carbon dioxide (CO 2 ) and carbon monoxide to hydrocarbons, offering a pathway to recycle carbon waste into hydrocarbon products. Among the three nitrogenase isozymes, the iron nitrogenase has the highest wild-type activity for the reduction of CO 2 , but the molecular architecture facilitating these activities has remained unknown. Here, we report a 2.35-Å cryogenic electron microscopy structure of the ADP·AlF 3 -stabilized iron nitrogenase complex from Rhodobacter capsulatus, revealing an [Fe 8 S 9 C-(R)-homocitrate] cluster in the active site. The enzyme complex suggests that the iron nitrogenase G subunit is involved in cluster stabilization and substrate channeling and confers specificity between nitrogenase reductase and catalytic component proteins. Moreover, the structure highlights a different interface between the two catalytic halves of the iron and the molybdenum nitrogenase, potentially influencing the intrasubunit 'communication' and thus the nitrogenase mechanism.
- Microbial Metalloenzymes Research Group, Max Planck Institute for Terrestrial Microbiology, Marburg, Germany.
Organizational Affiliation: