The RAGE and HMGB1 complex
Han, C.W., Kim, H.J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| High mobility group protein B1 | 75 | Homo sapiens | Mutation(s): 0 Gene Names: HMGB1, HMG1 | 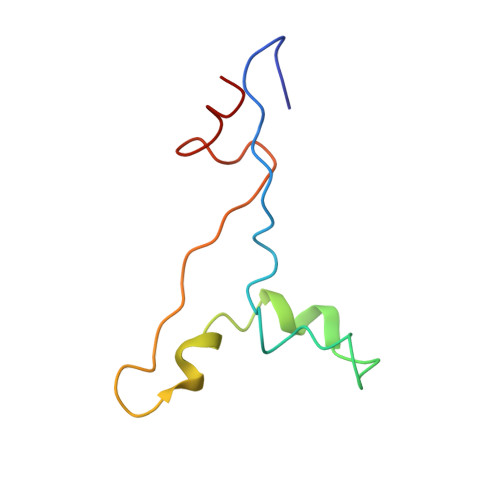 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P09429 (Homo sapiens) Explore P09429 Go to UniProtKB: P09429 | |||||
PHAROS: P09429 GTEx: ENSG00000189403 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P09429 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Advanced glycosylation end product-specific receptor | 211 | Homo sapiens | Mutation(s): 0 Gene Names: AGER, RAGE | 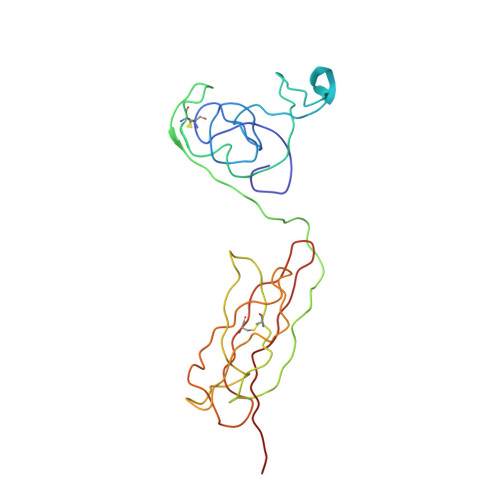 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q15109 (Homo sapiens) Explore Q15109 Go to UniProtKB: Q15109 | |||||
PHAROS: Q15109 GTEx: ENSG00000204305 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q15109 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |