SARS-CoV-2 variants of concern: spike protein mutational analysis and epitope for broad neutralization.
Mannar, D., Saville, J.W., Sun, Z., Zhu, X., Marti, M.M., Srivastava, S.S., Berezuk, A.M., Zhou, S., Tuttle, K.S., Sobolewski, M.D., Kim, A., Treat, B.R., Da Silva Castanha, P.M., Jacobs, J.L., Barratt-Boyes, S.M., Mellors, J.W., Dimitrov, D.S., Li, W., Subramaniam, S.(2022) Nat Commun 13: 4696-4696
- PubMed: 35982054
- DOI: https://doi.org/10.1038/s41467-022-32262-8
- Primary Citation of Related Structures:
8DLI, 8DLJ, 8DLK, 8DLL, 8DLM, 8DLN, 8DLO, 8DLP, 8DLQ, 8DLR, 8DLS, 8DLT, 8DLU, 8DLV, 8DLW, 8DLX, 8DLY, 8DLZ, 8DM0 - PubMed Abstract:
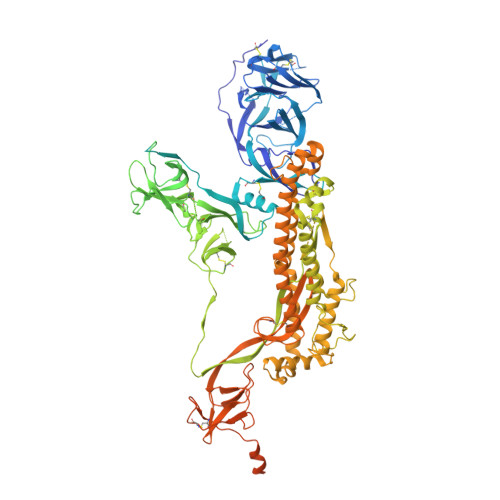
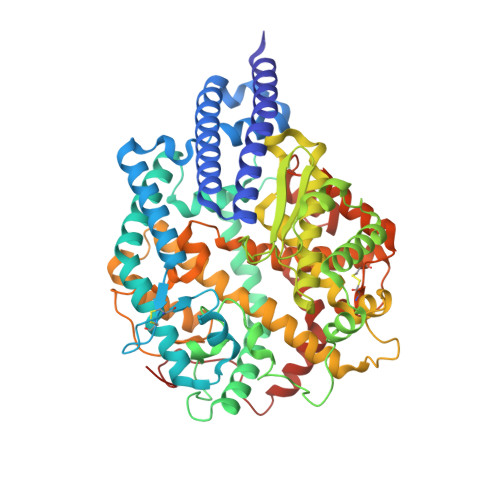
Mutations in the spike glycoproteins of SARS-CoV-2 variants of concern have independently been shown to enhance aspects of spike protein fitness. Here, we describe an antibody fragment (V H ab6) that neutralizes all major variants including the recently emerged BA.1 and BA.2 Omicron subvariants, with a unique mode of binding revealed by cryo-EM studies. Further, we provide a comparative analysis of the mutational effects within previously emerged variant spikes and identify the structural role of mutations within the NTD and RBD in evading antibody neutralization. Our analysis shows that the highly mutated Gamma N-terminal domain exhibits considerable structural rearrangements, partially explaining its decreased neutralization by convalescent sera. Our results provide mechanistic insights into the structural, functional, and antigenic consequences of SARS-CoV-2 spike mutations and highlight a spike protein vulnerability that may be exploited to achieve broad protection against circulating variants.
- Department of Biochemistry and Molecular Biology, University of British Columbia, Vancouver, BC, V6T 1Z3, Canada.
Organizational Affiliation: