The Crystal Structure of Thermal Green Protein Q66E (TGP-E) and Yellow Thermostable Protein (YTP-E) E148D
Anderson, M.R., Padgett, C.M., Ogbeifun, V.O., DeVore, N.M.(2024) SynBio 2: 298-310
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2024) SynBio 2: 298-310
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| extreme thermostable green fluorescent protein (TGP-E) | 249 | synthetic construct | Mutation(s): 0 | 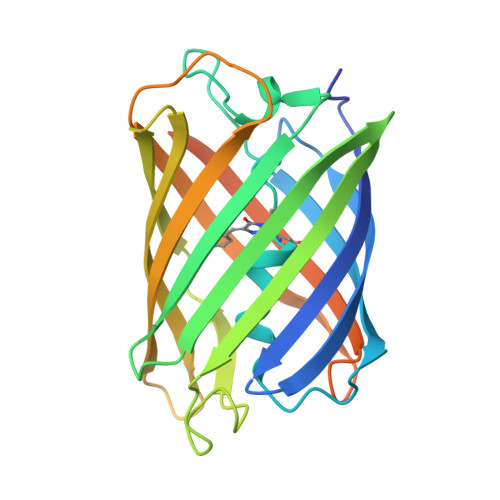 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CRU Query on CRU | A, B | L-PEPTIDE LINKING | C16 H15 N3 O6 |  | GLU, TYR, GLY |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 38.736 | α = 78.55 |
| b = 49.064 | β = 72.08 |
| c = 59.108 | γ = 71.41 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| REFMAC | refinement |
| CrysalisPro | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Science Foundation (NSF, United States) | United States | 2117129 |