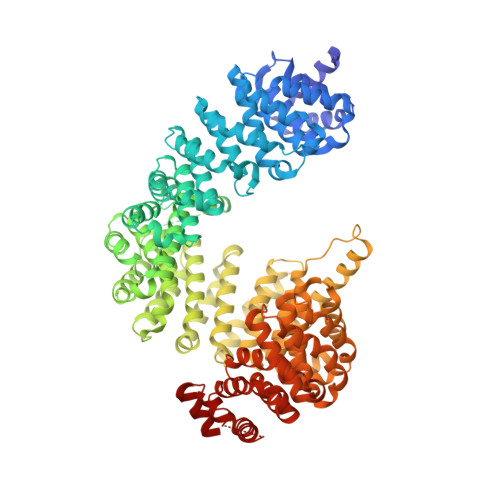
A new Karyopherin-beta 2 binding PY-NLS epitope of HNRNPH2 linked to neurodevelopmental disorders.
Gonzalez, A., Kim, H.J., Freibaum, B.D., Fung, H.Y.J., Brautigam, C.A., Taylor, J.P., Chook, Y.M.(2023) Structure 31: 924-934.e4
- PubMed: 37279758
- DOI: https://doi.org/10.1016/j.str.2023.05.010
- Primary Citation of Related Structures:
8SGH - PubMed Abstract:
The HNRNPH2 proline-tyrosine nuclear localization signal (PY-NLS) is mutated in HNRNPH2-related X-linked neurodevelopmental disorder, causing the normally nuclear HNRNPH2 to accumulate in the cytoplasm. We solved the cryoelectron microscopy (cryo-EM) structure of Karyopherin-β2/Transportin-1 bound to the HNRNPH2 PY-NLS to understand importin-NLS recognition and disruption in disease. HNRNPH2 206 RPGPY 210 is a typical R-X 2-4 -P-Y motif comprising PY-NLS epitopes 2 and 3, followed by an additional Karyopherin-β2-binding epitope, we term epitope 4, at residues 211 DRP 213 ; no density is present for PY-NLS epitope 1. Disease variant mutations at epitopes 2-4 impair Karyopherin-β2 binding and cause aberrant cytoplasmic accumulation in cells, emphasizing the role of nuclear import defect in disease. Sequence/structure analysis suggests that strong PY-NLS epitopes 4 are rare and thus far limited to close paralogs of HNRNPH2, HNRNPH1, and HNRNPF. Epitope 4-binidng hotspot Karyopherin-β2 W373 corresponds to close paralog Karyopherin-β2b/Transportin-2 W370, a pathological variant site in neurodevelopmental abnormalities, suggesting that Karyopherin-β2b/Transportin-2-HNRNPH2/H1/F interactions may be compromised in the abnormalities.
- Department of Pharmacology, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Organizational Affiliation: