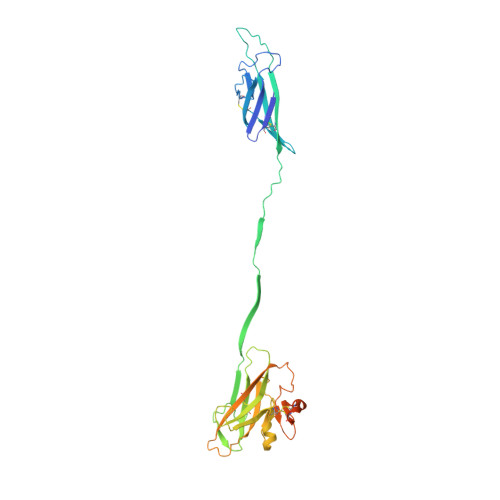
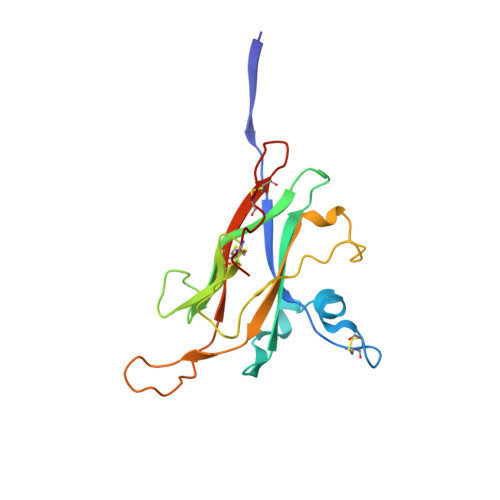
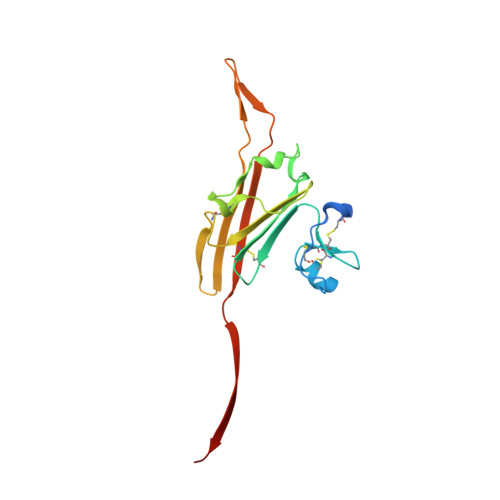
ZP2 cleavage blocks polyspermy by modulating the architecture of the egg coat.
Nishio, S., Emori, C., Wiseman, B., Fahrenkamp, D., Dioguardi, E., Zamora-Caballero, S., Bokhove, M., Han, L., Stsiapanava, A., Algarra, B., Lu, Y., Kodani, M., Bainbridge, R.E., Komondor, K.M., Carlson, A.E., Landreh, M., de Sanctis, D., Yasumasu, S., Ikawa, M., Jovine, L.(2024) Cell 187: 1440-1459.e24
- PubMed: 38490181
- DOI: https://doi.org/10.1016/j.cell.2024.02.013
- Primary Citation of Related Structures:
8BQU, 8RKE, 8RKF, 8RKG, 8RKH, 8RKI - PubMed Abstract:
Following the fertilization of an egg by a single sperm, the egg coat or zona pellucida (ZP) hardens and polyspermy is irreversibly blocked. These events are associated with the cleavage of the N-terminal region (NTR) of glycoprotein ZP2, a major subunit of ZP filaments. ZP2 processing is thought to inactivate sperm binding to the ZP, but its molecular consequences and connection with ZP hardening are unknown. Biochemical and structural studies show that cleavage of ZP2 triggers its oligomerization. Moreover, the structure of a native vertebrate egg coat filament, combined with AlphaFold predictions of human ZP polymers, reveals that two protofilaments consisting of type I (ZP3) and type II (ZP1/ZP2/ZP4) components interlock into a left-handed double helix from which the NTRs of type II subunits protrude. Together, these data suggest that oligomerization of cleaved ZP2 NTRs extensively cross-links ZP filaments, rigidifying the egg coat and making it physically impenetrable to sperm.
- Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, Sweden.
Organizational Affiliation: