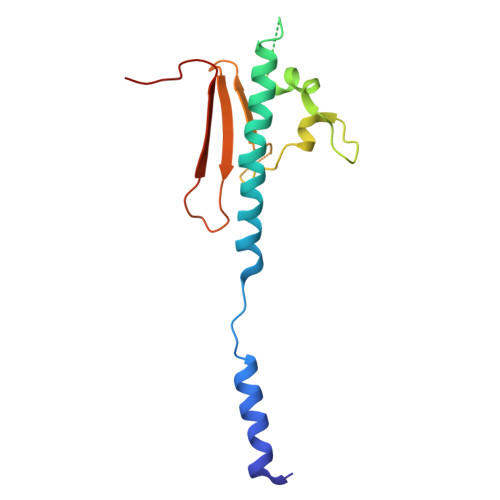
Structure of a heteropolymeric type 4 pilus from a monoderm bacterium.
Anger, R., Pieulle, L., Shahin, M., Valette, O., Le Guenno, H., Kosta, A., Pelicic, V., Fronzes, R.(2023) Nat Commun 14: 7143-7143
- PubMed: 37932265
- DOI: https://doi.org/10.1038/s41467-023-42872-5
- Primary Citation of Related Structures:
8PFB - PubMed Abstract:
Type 4 pili (T4P) are important virulence factors, which belong to a superfamily of nanomachines ubiquitous in prokaryotes, called type 4 filaments (T4F). T4F are defined as helical polymers of type 4 pilins. Recent advances in cryo-electron microscopy (cryo-EM) led to structures of several T4F, revealing that the long N-terminal α-helix (α1) - the trademark of pilins - packs in the centre of the filaments to form a hydrophobic core. In diderm bacteria - all available bacterial T4F structures are from diderm species - a portion of α1 is melted (unfolded). Here we report that this architecture is conserved in phylogenetically distant monoderm species by determining the structure of Streptococcus sanguinis T4P. Our 3.7 Å resolution cryo-EM structure of S. sanguinis heteropolymeric T4P and the resulting full atomic model including all minor pilins highlight universal features of bacterial T4F and have widespread implications in understanding T4F biology.
- Institut Européen de Chimie et Biologie, Université de Bordeaux-CNRS (UMR 5234), Pessac, France.
Organizational Affiliation: