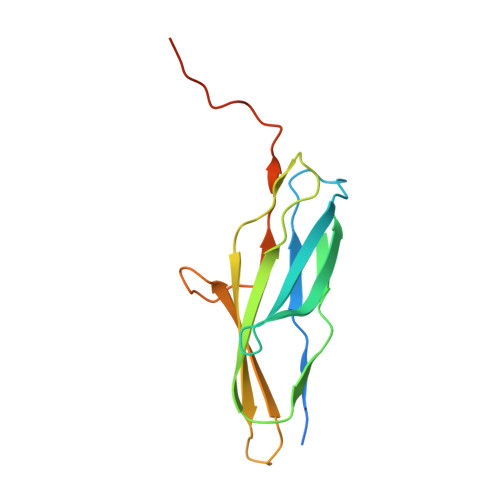
Analysis of FctB3 crystal structure and insight into its structural stabilization and pilin linkage mechanisms.
Takebe, K., Suzuki, M., Sangawa, T., Kreikemeyer, B., Yamaguchi, M., Uzawa, N., Sumitomo, T., Kawabata, S., Nakata, M.(2023) Arch Microbiol 206: 4-4
- PubMed: 37994962
- DOI: https://doi.org/10.1007/s00203-023-03727-1
- Primary Citation of Related Structures:
8K6T - PubMed Abstract:
Streptococcus pyogenes harboring an FCT type 3 genomic region display pili composed of three types of pilins. In this study, the structure of the base pilin FctB from a serotype M3 strain (FctB3) was determined at 2.8 Å resolution. In accordance with the previously reported structure of FctB from a serotype T9 strain (FctB9), FctB3 was found to consist of an immunoglobulin-like domain and proline-rich tail region. Data obtained from structure comparison revealed main differences in the omega (Ω) loop structure and the proline-rich tail direction. In the Ω loop structure, a differential hydrogen bond network was observed, while the lysine residue responsible for linkage to growing pili was located at the same position in both structures, which indicated that switching of the hydrogen bond network in the Ω loop without changing the lysine position is advantageous for linkage to the backbone pilin FctA. The difference in direction of the proline-rich tail is potentially caused by a single residue located at the root of the proline-rich tail. Also, the FctB3 structure was found to be stabilized by intramolecular large hydrophobic interactions instead of an isopeptide bond. Comparisons of the FctB3 and FctA structures indicated that the FctA structure is more favorable for linkage to FctA. In addition, the heterodimer formation of FctB with Cpa or FctA was shown to be mediated by the putative chaperone SipA. Together, these findings provide an alternative FctB structure as well as insight into the interactions between pilin proteins.
- Department of Oral and Maxillofacial Oncology and Surgery, Osaka University Graduate School of Dentistry, 1-8, Yamadaoka, Suita, Osaka, Japan.
Organizational Affiliation: