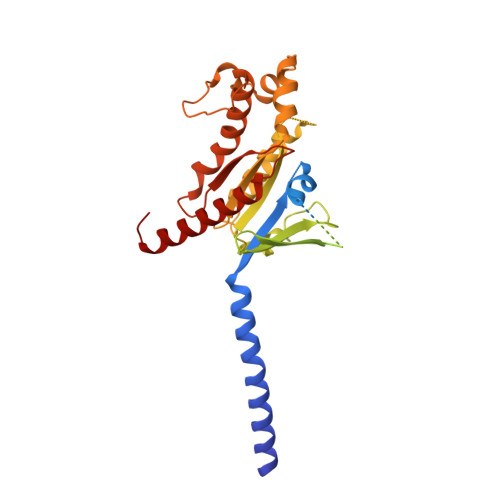
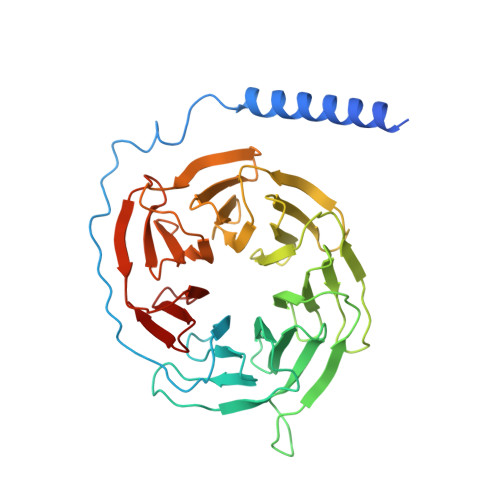
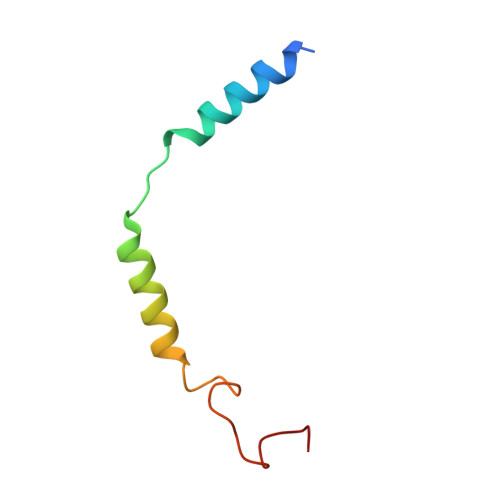
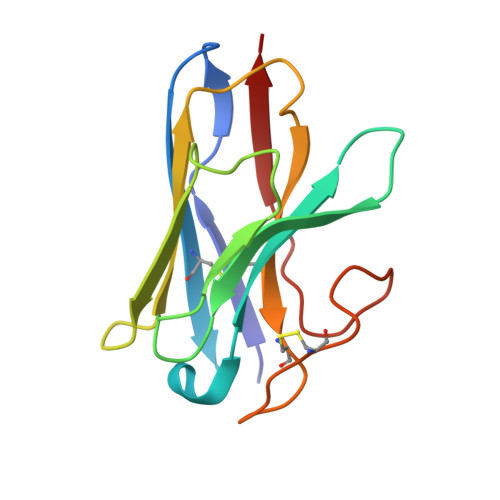
Structural insight into the selective agonist ST1936 binding of serotonin receptor 5-HT6.
Pei, Y., Wen, X., Guo, S.C., Yang, Z.S., Zhang, R., Xiao, P., Sun, J.P.(2023) Biochem Biophys Res Commun 671: 327-334
- PubMed: 37327704
- DOI: https://doi.org/10.1016/j.bbrc.2023.05.126
- Primary Citation of Related Structures:
8JLZ - PubMed Abstract:
The serotonin receptor 5-HT 6 R is an important G-protein-coupled receptor (GPCR) that involved in essential functions within the central and peripheral nervous systems and is linked to various psychiatric disorders. Selective activation of 5-HT 6 R promotes neural stem cell regeneration activity. As a 5-HT 6 R selective agonist, 2-(5 chloro-2-methyl-1H-indol-3-yl)-N, N-dimethylethanolamine (ST1936) has been widely used to investigate the functions of the 5-HT 6 R. The molecular mechanism of how ST1936 is recognized by 5-HT 6 R and how it effectively couples with Gs remain unclear. Here, we reconstituted the ST1936-5-HT 6 R-Gs complex in vitro and solved its cryo-electron microscopy structure at 3.1 Å resolution. Further structural analysis and mutational studies facilitated us to identify the residues of the Y310 7.43 and "toggle switch" W281 6.48 of the 5-HT 6 R contributed to the higher efficacy of ST1936 compared with 5-HT. By uncovering the structural foundation of how 5-HT 6 R specifically recognizes agonists and elucidating the molecular process of G protein activation, our discoveries offer valuable insights and pave the way for the development of promising 5-HT 6 R agonists.
- State Key Laboratory of Surface Physics, Key Laboratory of Micro and Nano Photonic Structures (Ministry of Education) and Collaborative Innovation Center of Advanced Microstructures, Fudan University, Shanghai, 200433, China; Advanced Medical Research Institute, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, 250012, China.
Organizational Affiliation: