Carotenoid assembly regulates quinone diffusion and the Roseiflexus castenholzii reaction center-light harvesting complex architecture.
Xin, J., Shi, Y., Zhang, X., Yuan, X., Xin, Y., He, H., Shen, J., Blankenship, R.E., Xu, X.(2023) Elife 12
- PubMed: 37737710
- DOI: https://doi.org/10.7554/eLife.88951
- Primary Citation of Related Structures:
8HJU, 8HJV, 8J5O, 8J5P - PubMed Abstract:
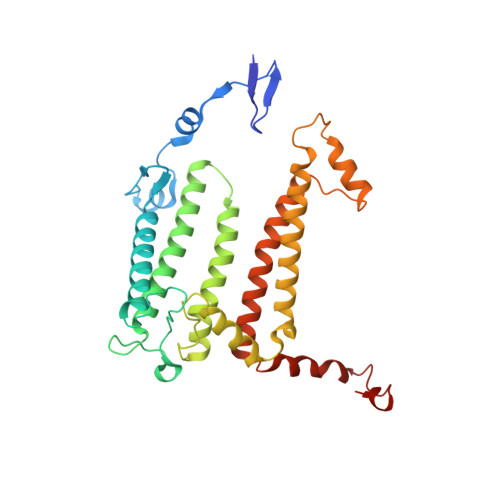
Carotenoid (Car) pigments perform central roles in photosynthesis-related light harvesting (LH), photoprotection, and assembly of functional pigment-protein complexes. However, the relationships between Car depletion in the LH, assembly of the prokaryotic reaction center (RC)-LH complex, and quinone exchange are not fully understood. Here, we analyzed native RC-LH (nRC-LH) and Car-depleted RC-LH (dRC-LH) complexes in Roseiflexus castenholzii , a chlorosome-less filamentous anoxygenic phototroph that forms the deepest branch of photosynthetic bacteria. Newly identified exterior Cars functioned with the bacteriochlorophyll B800 to block the proposed quinone channel between LHαβ subunits in the nRC-LH, forming a sealed LH ring that was disrupted by transmembrane helices from cytochrome c and subunit X to allow quinone shuttling. dRC-LH lacked subunit X, leading to an exposed LH ring with a larger opening, which together accelerated the quinone exchange rate. We also assigned amino acid sequences of subunit X and two hypothetical proteins Y and Z that functioned in forming the quinone channel and stabilizing the RC-LH interactions. This study reveals the structural basis by which Cars assembly regulates the architecture and quinone exchange of bacterial RC-LH complexes. These findings mark an important step forward in understanding the evolution and diversity of prokaryotic photosynthetic apparatus.
- Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences and The Affiliated Hospital of Hangzhou Normal University, Hangzhou, China.
Organizational Affiliation: