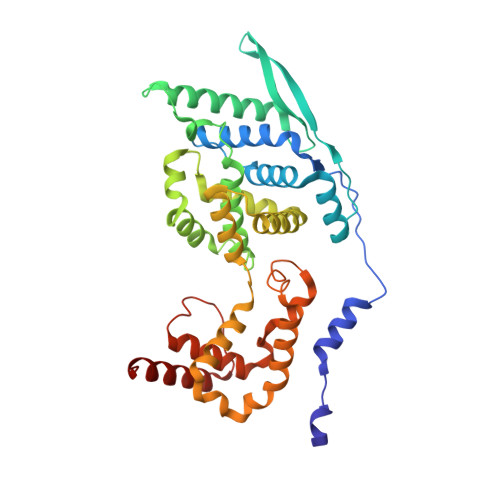
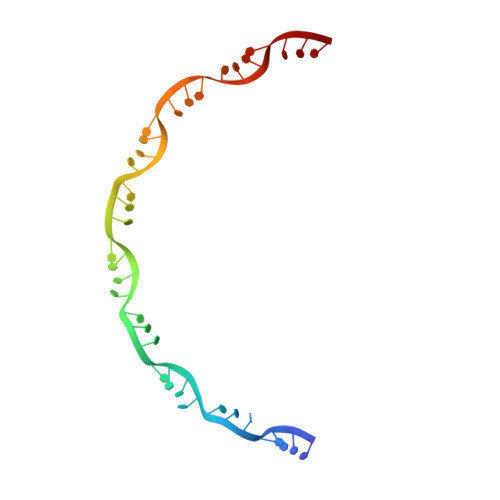Cryo-EM structure of the nucleocapsid-like assembly of respiratory syncytial virus.
Wang, Y., Zhang, C., Luo, Y., Ling, X., Luo, B., Jia, G., Su, D., Dong, H., Su, Z.(2023) Signal Transduct Target Ther 8: 323-323
- PubMed: 37607909
- DOI: https://doi.org/10.1038/s41392-023-01602-5
- Primary Citation of Related Structures:
8IUO - PubMed Abstract:
Respiratory syncytial virus (RSV) is a nonsegmented, negative strand RNA virus that has caused severe lower respiratory tract infections of high mortality rates in infants and the elderly, yet no effective vaccine or antiviral therapy is available. The RSV genome encodes the nucleoprotein (N) that forms helical assembly to encapsulate and protect the RNA genome from degradation, and to serve as a template for transcription and replication. Previous crystal structure revealed a decameric ring architecture of N in complex with the cellular RNA (N-RNA) of 70 nucleotides (70-nt), whereas cryo-ET reconstruction revealed a low-resolution left-handed filament, in which the crystal monomer structure was docked with the helical symmetry applied to simulate a nucleocapsid-like assembly of RSV. However, the molecular details of RSV nucleocapsid assembly remain unknown, which continue to limit our complete understanding of the critical interactions involved in the nucleocapsid and antiviral development that may target this essential process during the viral life cycle. Here we resolve the near-atomic cryo-EM structure of RSV N-RNA that represents roughly one turn of the helical assembly that unveils critical interaction interfaces of RSV nucleocapsid and may facilitate development of RSV antiviral therapy.
- The State Key Laboratory of Biotherapy, Frontiers Medical Center of Tianfu Jincheng Laboratory, Department of Geriatrics and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, Sichuan, 610044, China.
Organizational Affiliation: