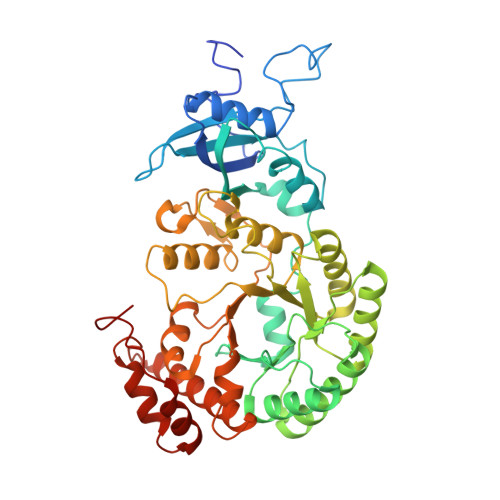
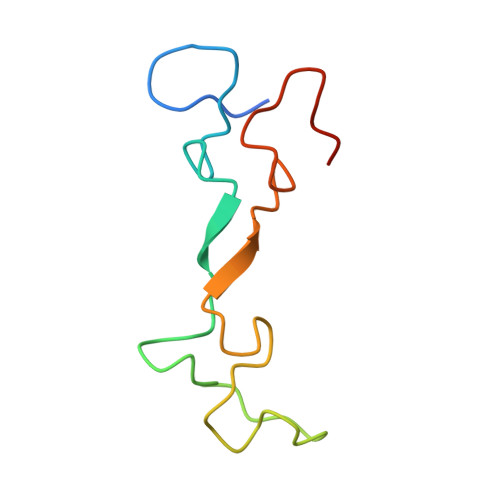
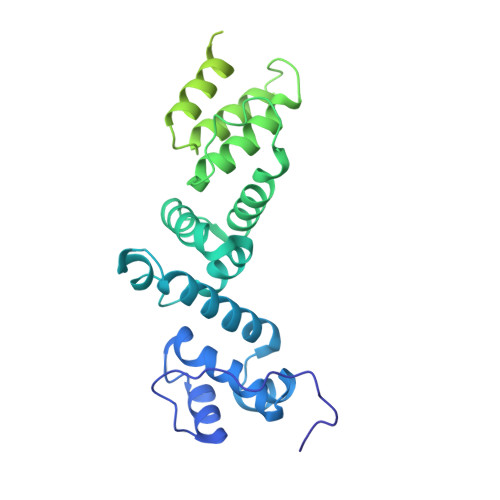
Structural insights into the functions of Raf1 and Bsd2 in hexadecameric Rubisco assembly.
Wang, R., Song, H., Zhang, W., Wang, N., Zhang, S., Shao, R., Liu, C.(2023) Mol Plant 16: 1927-1936
- PubMed: 37853692
- DOI: https://doi.org/10.1016/j.molp.2023.10.011
- Primary Citation of Related Structures:
8ILB, 8ILM, 8IO2, 8IOJ, 8IOL - PubMed Abstract:
Hexadecameric form I Rubisco, which consisting consists of eight large (RbcL) and eight small (RbcS) subunits, is the most abundant enzyme on earth. Extensive efforts to engineer an improved Rubisco to speed up its catalytic efficiency and ultimately increase agricultural productivity. However, difficulties with correct folding and assembly in foreign hosts or in vitro have hampered the genetic manipulation of hexadecameric Rubisco. In this study, we reconstituted Synechococcus sp. PCC6301 Rubisco in vitro using the chaperonin system and assembly factors from cyanobacteria and Arabidopsis thaliana (At). Rubisco holoenzyme was produced in the presence of cyanobacterial Rubisco accumulation factor 1 (Raf1) alone or both AtRaf1 and bundle-sheath defective-2 (AtBsd2) from Arabidopsis. RbcL released from GroEL is assembly capable in the presence of ATP, and AtBsd2 functions downstream of AtRaf1. Cryo-EM structures of RbcL 8 -AtRaf1 8 , RbcL 8 -AtRaf1 4 -AtBsd2 8 , and RbcL 8 revealed that the interactions between RbcL and AtRaf1 are looser than those between prokaryotic RbcL and Raf1, with AtRaf1 tilting 7° farther away from RbcL. AtBsd2 stabilizes the flexible regions of RbcL, including the N and C termini, the 60s loop, and loop 6. Using these data, combined with previous findings, we propose the possible biogenesis pathways of prokaryotic and eukaryotic Rubisco.
- State Key Laboratory of Plant Cell and Chromosome Engineering, Institute of Genetics and Developmental Biology, The Innovative Academy of Seed Design, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100101, China.
Organizational Affiliation: