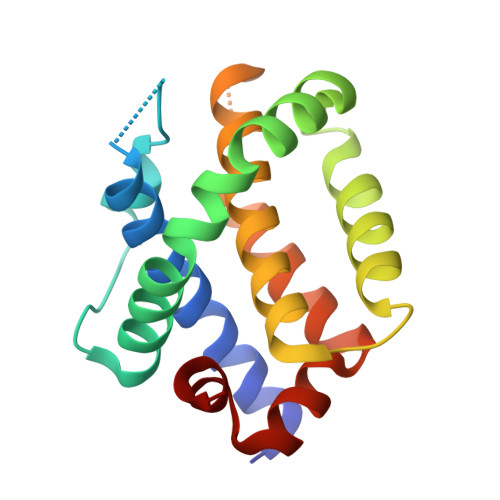
Crystal structure of Bak bound to the BH3 domain of Bnip5, a noncanonical BH3 domain-containing protein.
Lim, D., Jeong, D.E., Shin, H.C., Choi, J.S., Seo, J., Kim, S.J., Ku, B.(2024) Proteins 92: 44-51
- PubMed: 37553948
- DOI: https://doi.org/10.1002/prot.26568
- Primary Citation of Related Structures:
8IGC - PubMed Abstract:
The activation or inactivation of B-cell lymphoma-2 (Bcl-2) antagonist/killer (Bak) is critical for controlling mitochondrial outer membrane permeabilization-dependent apoptosis. Its pro-apoptotic activity is controlled by intermolecular interactions with the Bcl-2 homology 3 (BH3) domain, which is accommodated in the hydrophobic pocket of Bak. Bcl-2-interacting protein 5 (Bnip5) is a noncanonical BH3 domain-containing protein that interacts with Bak. Bnip5 is characterized by its controversial effects on the regulation of the pro-apoptotic activity of Bak. In the present study, we determined the crystal structure of Bak bound to Bnip5 BH3. The intermolecular association appeared to be typical at first glance, but we found that it is maintained by tight hydrophobic interactions together with hydrogen/ionic bonds, which accounts for their high binding affinity with a dissociation constant of 775 nM. Structural analysis of the complex showed that Bnip5 interacts with Bak in a manner similar to that of the Bak-activating pro-apoptotic factor peroxisomal testis-enriched protein 1, particularly in the destabilization of the intramolecular electrostatic network of Bak. Our structure is considered to reflect the initial point of drastic and consecutive conformational and stoichiometric changes in Bak induced by Bnip5 BH3, which helps in explaining the effects of Bnip5 in regulating Bak-mediated apoptosis.
- Disease Target Structure Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon, Republic of Korea.
Organizational Affiliation: