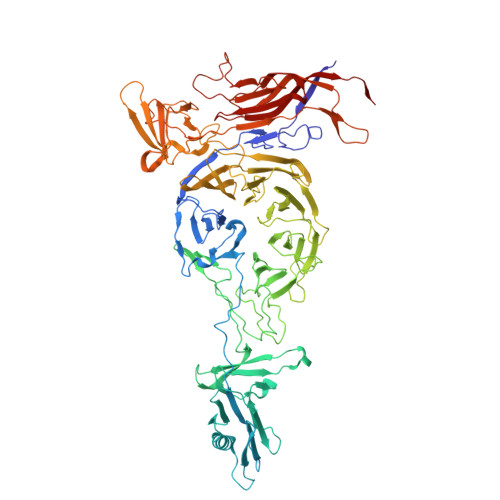
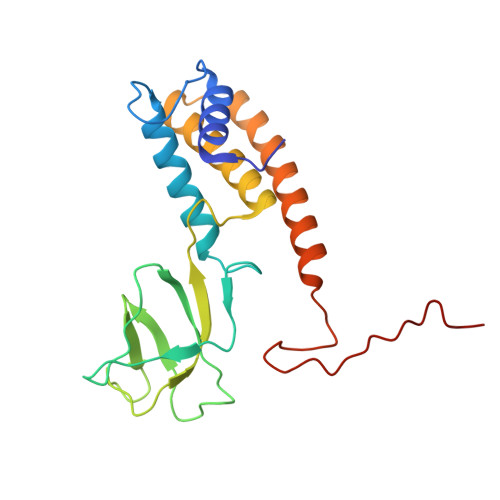
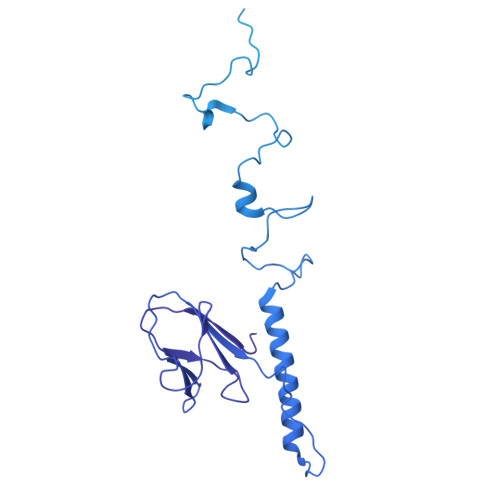
Cryo-EM structure of cyanophage P-SCSP1u offers insights into DNA gating and evolution of T7-like viruses.
Cai, L., Liu, H., Zhang, W., Xiao, S., Zeng, Q., Dang, S.(2023) Nat Commun 14: 6438-6438
- PubMed: 37833330
- DOI: https://doi.org/10.1038/s41467-023-42258-7
- Primary Citation of Related Structures:
8I4L, 8I4M - PubMed Abstract:
Cyanophages, together with their host cyanobacteria, play important roles in marine biogeochemical cycles and control of marine food webs. The recently identified MPP-C (Marine Picocyanobacteria Podovirus clade C) cyanophages, belonging to the T7-like podoviruses, contain the smallest genomes among cyanopodoviruses and exhibit distinct infection kinetics. However, understanding of the MPP-C cyanophage infection process is hindered by the lack of high-resolution structural information. Here, we report the cryo-EM structure of the cyanophage P-SCSP1u, a representative member of the MPP-C phages, in its native form at near-atomic resolution, which reveals the assembly mechanism of the capsid and molecular interaction of the portal-tail complex. Structural comparison of the capsid proteins of P-SCSP1u and other podoviruses with known structures provides insights into the evolution of T7-like viruses. Furthermore, our study provides the near-atomic resolution structure of portal-tail complex for T7-like viruses. On the basis of previously reported structures of phage T7, we identify an additional valve and gate to explain the DNA gating mechanism for the T7-like viruses.
- Department of Ocean Science, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, China.
Organizational Affiliation: