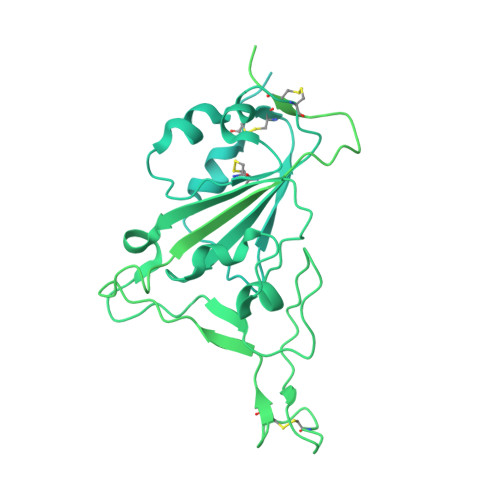
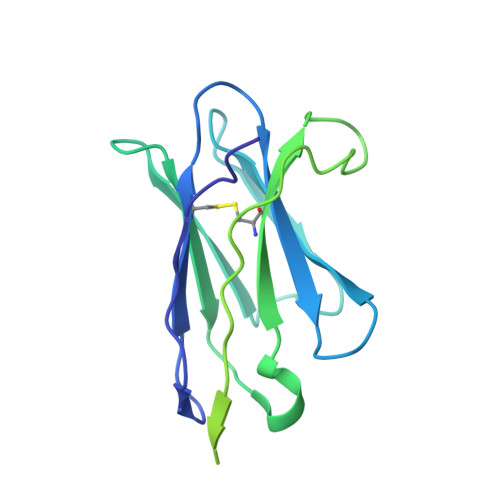
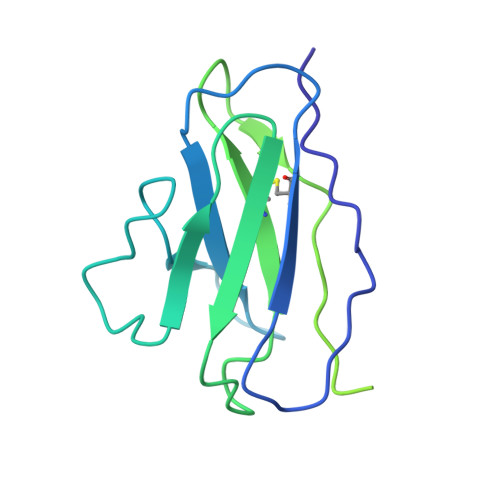
Broadly neutralizing antibodies derived from the earliest COVID-19 convalescents protect mice from SARS-CoV-2 variants challenge.
Liu, Q., Zhao, H., Li, Z., Zhang, Z., Huang, R., Gu, M., Zhuang, K., Xiong, Q., Chen, X., Yu, W., Qian, S., Zhang, Y., Tan, X., Zhang, M., Yu, F., Guo, M., Huang, Z., Wang, X., Xiang, W., Wu, B., Mei, F., Cai, K., Zhou, L., Zhou, L., Wu, Y., Yan, H., Cao, S., Lan, K., Chen, Y.(2023) Signal Transduct Target Ther 8: 347-347
- PubMed: 37704615
- DOI: https://doi.org/10.1038/s41392-023-01615-0
- Primary Citation of Related Structures:
8I3S, 8I3U - PubMed Abstract:
Coronavirus disease 2019 (COVID-19) was first reported three years ago, when a group of individuals were infected with the original SARS-CoV-2 strain, based on which vaccines were developed. Here, we develop six human monoclonal antibodies (mAbs) from two elite convalescents in Wuhan and show that these mAbs recognize diverse epitopes on the receptor binding domain (RBD) and can inhibit the infection of SARS-CoV-2 original strain and variants of concern (VOCs) to varying degrees, including Omicron strains XBB and XBB.1.5. Of these mAbs, the two most broadly and potently neutralizing mAbs (7B3 and 14B1) exhibit prophylactic activity against SARS-CoV-2 WT infection and therapeutic effects against SARS-CoV-2 Delta variant challenge in K18-hACE2 KI mice. Furthermore, post-exposure treatment with 7B3 protects mice from lethal Omicron variants infection. Cryo-EM analysis of the spike trimer complexed with 14B1 or 7B3 reveals that these two mAbs bind partially overlapped epitopes onto the RBD of the spike, and sterically disrupt the binding of human angiotensin-converting enzyme 2 (hACE2) to RBD. Our results suggest that mAbs with broadly neutralizing activity against different SARS-CoV-2 variants are present in COVID-19 convalescents infected by the ancestral SARS-CoV-2 strain, indicating that people can benefit from former infections or vaccines despite the extensive immune escape of SARS-CoV-2.
- State Key Laboratory of Virology, Institute for Vaccine Research, College of Life Sciences, Wuhan University, Wuhan, 430072, China.
Organizational Affiliation: