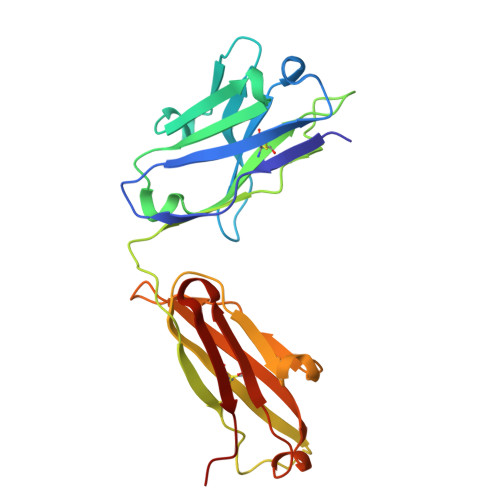
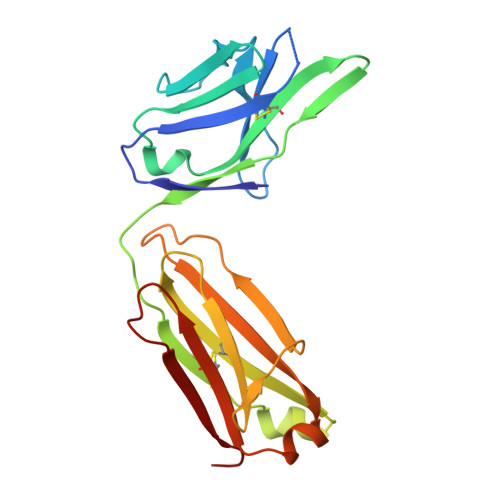
XTX101, a tumor-activated, Fc-enhanced anti-CTLA-4 monoclonal antibody, demonstrates tumor-growth inhibition and tumor-selective pharmacodynamics in mouse models of cancer.
Jenkins, K.A., Park, M., Pederzoli-Ribeil, M., Eskiocak, U., Johnson, P., Guzman, W., McLaughlin, M., Moore-Lai, D., O'Toole, C., Liu, Z., Nicholson, B., Flesch, V., Qiu, H., Clackson, T., O'Hagan, R.C., Rodeck, U., Karow, M., O'Neil, J., Williams, J.C.(2023) J Immunother Cancer 11
- PubMed: 38164757
- DOI: https://doi.org/10.1136/jitc-2023-007785
- Primary Citation of Related Structures:
8G2M, 8G8N - PubMed Abstract:
The clinical benefit of the anti-CTLA-4 monoclonal antibody (mAb) ipilimumab has been well established but limited by immune-related adverse events, especially when ipilimumab is used in combination with anti-PD-(L)1 mAb therapy. To overcome these limitations, we have developed XTX101, a tumor-activated, Fc-enhanced anti-CTLA-4 mAb. XTX101 consists of an anti-human CTLA-4 mAb covalently linked to masking peptides that block the complementarity-determining regions, thereby minimizing the mAb binding to CTLA-4. The masking peptides are designed to be released by proteases that are typically dysregulated within the tumor microenvironment (TME), resulting in activation of XTX101 intratumorally. Mutations within the Fc region of XTX101 were included to enhance affinity for FcγRIII, which is expected to enhance potency through antibody-dependent cellular cytotoxicity. Biophysical, biochemical, and cell-based assays demonstrate that the function of XTX101 depends on proteolytic activation. In human CTLA-4 transgenic mice, XTX101 monotherapy demonstrated significant tumor growth inhibition (TGI) including complete responses, increased intratumoral CD8+T cells, and regulatory T cell depletion within the TME while maintaining minimal pharmacodynamic effects in the periphery. XTX101 in combination with anti-PD-1 mAb treatment resulted in significant TGI and was well tolerated in mice. XTX101 was activated in primary human tumors across a range of tumor types including melanoma, renal cell carcinoma, colon cancer and lung cancer in an ex vivo assay system. These data demonstrate that XTX101 retains the full potency of an Fc-enhanced CTLA-4 antagonist within the TME while minimizing the activity in non-tumor tissue, supporting the further evaluation of XTX101 in clinical studies.
- Xilio Therapeutics, Waltham, Massachusetts, USA kjenkins@xiliotx.com.
Organizational Affiliation: