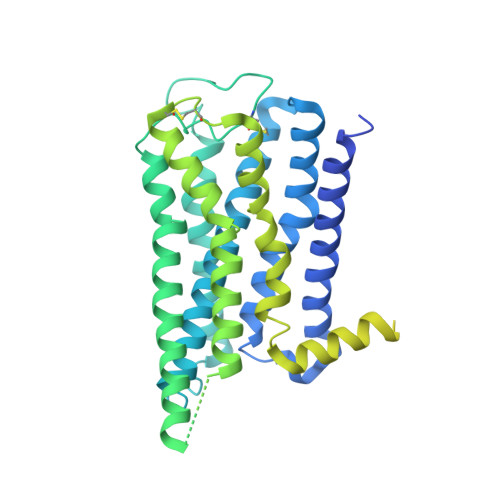
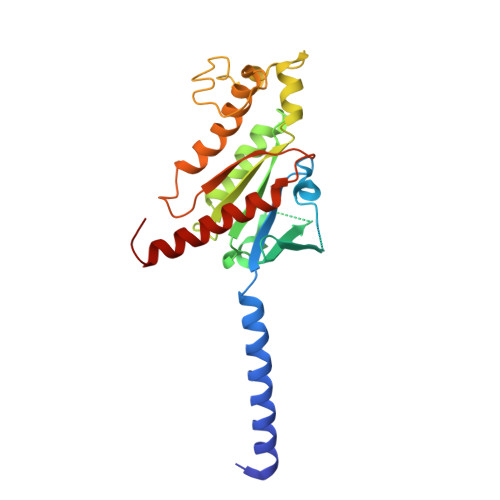
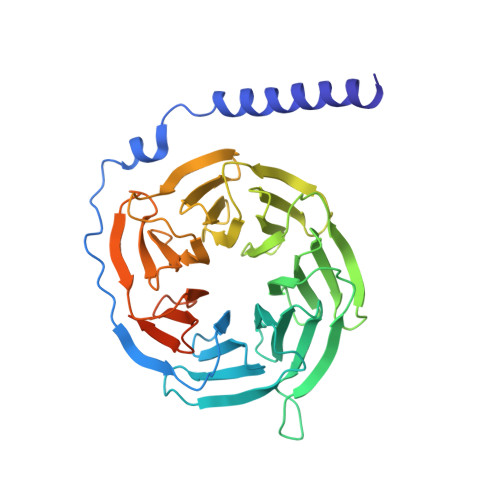
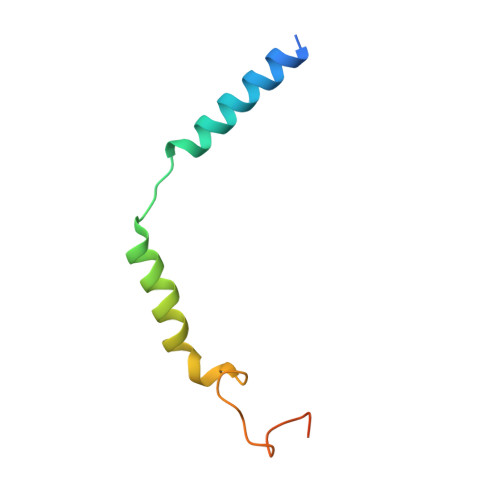
Molecular basis for selective activation of DREADD-based chemogenetics.
Zhang, S., Gumpper, R.H., Huang, X.P., Liu, Y., Krumm, B.E., Cao, C., Fay, J.F., Roth, B.L.(2022) Nature 612: 354-362
- PubMed: 36450989
- DOI: https://doi.org/10.1038/s41586-022-05489-0
- Primary Citation of Related Structures:
8E9W, 8E9X, 8E9Y, 8E9Z, 8EA0 - PubMed Abstract:
Designer receptors exclusively activated by designer drugs (DREADDs) represent a powerful chemogenetic technology for the remote control of neuronal activity and cellular signalling 1-4 . The muscarinic receptor-based DREADDs are the most widely used chemogenetic tools in neuroscience research. The G q -coupled DREADD (hM3Dq) is used to enhance neuronal activity, whereas the G i/o -coupled DREADD (hM4Di) is utilized to inhibit neuronal activity 5 . Here we report four DREADD-related cryogenic electron microscopy high-resolution structures: a hM3Dq-miniG q complex and a hM4Di-miniG o complex bound to deschloroclozapine; a hM3Dq-miniG q complex bound to clozapine-N-oxide; and a hM3R-miniG q complex bound to iperoxo. Complemented with mutagenesis, functional and computational simulation data, our structures reveal key details of the recognition of DREADD chemogenetic actuators and the molecular basis for activation. These findings should accelerate the structure-guided discovery of next-generation chemogenetic tools.
- Department of Pharmacology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Organizational Affiliation: