Harnessing Aromatic-Histidine Interactions through Synergistic Backbone Extension and Side Chain Modification.
Yu, Z., Kreitler, D.F., Chiu, Y.T.T., Xu, R., Bruchs, A.T., Bingman, C.A., Gellman, S.H.(2023) Angew Chem Int Ed Engl 62: e202308100-e202308100
- PubMed: 37587780
- DOI: https://doi.org/10.1002/anie.202308100
- Primary Citation of Related Structures:
8D51, 8D52 - PubMed Abstract:
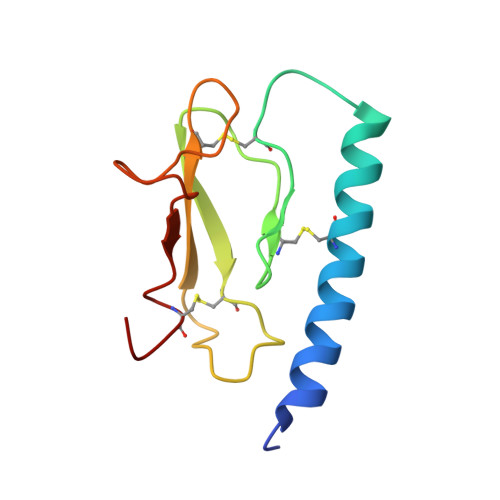
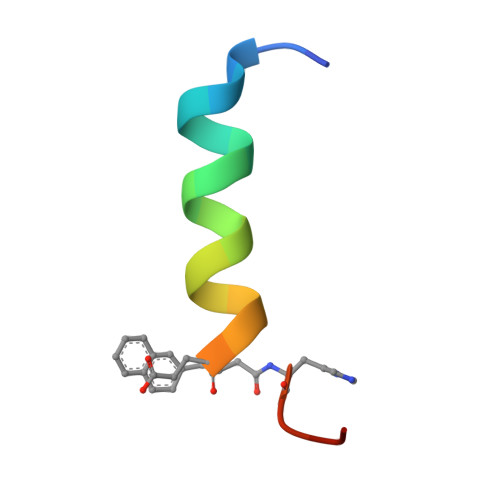
Peptide engineering efforts have delivered drugs for diverse human diseases. Side chain alteration is among the most common approaches to designing new peptides for specific applications. The peptide backbone can be modified as well, but this strategy has received relatively little attention. Here we show that new and favorable contacts between a His side chain on a target protein and an aromatic side chain on a synthetic peptide ligand can be engineered by rational and coordinated side chain modification and backbone extension. Side chain modification alone was unsuccessful. Binding measurements, high-resolution structural studies and pharmacological outcomes all support the synergy between backbone and side chain modification in engineered ligands of the parathyroid hormone receptor-1, which is targeted by osteoporosis drugs. These results should motivate other structure-based designs featuring coordinated side chain modification and backbone extension to enhance the engagement of peptide ligands with target proteins.
- Department of Chemistry, University of Wisconsin-Madison, Madison, Wisconsin, 53706, USA.
Organizational Affiliation: