Novel insecticidal proteins from ferns resemble insecticidal proteins from Bacillus thuringiensis.
Wei, J.Z., Lum, A., Schepers, E., Liu, L., Weston, R.T., McGinness, B.S., Heckert, M.J., Xie, W., Kassa, A., Bruck, D., Rauscher, G., Kapka-Kitzman, D., Mathis, J.P., Zhao, J.Z., Sethi, A., Barry, J., Lu, A.L., Brugliera, F., Lee, E.L., van derWeerden, N.L., Eswar, N., Maher, M.J., Anderson, M.A.(2023) Proc Natl Acad Sci U S A 120: e2306177120-e2306177120
- PubMed: 37871210
- DOI: https://doi.org/10.1073/pnas.2306177120
- Primary Citation of Related Structures:
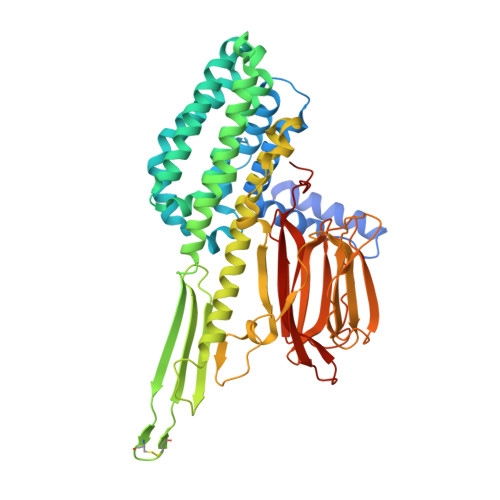
8D2J - PubMed Abstract:
Lepidopterans affect crop production worldwide. The use of transgenes encoding insecticidal proteins from Bacillus thuringiensis ( Bt ) in crop plants is a well-established technology that enhances protection against lepidopteran larvae. Concern about widespread field-evolved resistance to Bt proteins has highlighted an urgent need for new insecticidal proteins with different modes or sites of action. We discovered a new family of insecticidal proteins from ferns. The prototype protein from Pteris species (Order Polypodiales) and variants from two other orders of ferns, Schizaeales and Ophioglossales, were effective against important lepidopteran pests of maize and soybean in diet-based assays. Transgenic maize and soybean plants producing these proteins were more resistant to insect damage than controls. We report here the crystal structure of a variant of the prototype protein to 1.98 Å resolution. Remarkably, despite being derived from plants, the structure resembles the 3-domain Cry proteins from Bt but has only two out of three of their characteristic domains, lacking the C-terminal domain which is typically required for their activities. Two of the fern proteins were effective against strains of fall armyworm that were resistant to Bt 3-domain Cry proteins Cry1Fa or Cry2A.127. This therefore represents a novel family of insecticidal proteins that have the potential to provide future tools for pest control.
- Corteva Agriscience, Johnston, IA 50131.
Organizational Affiliation: