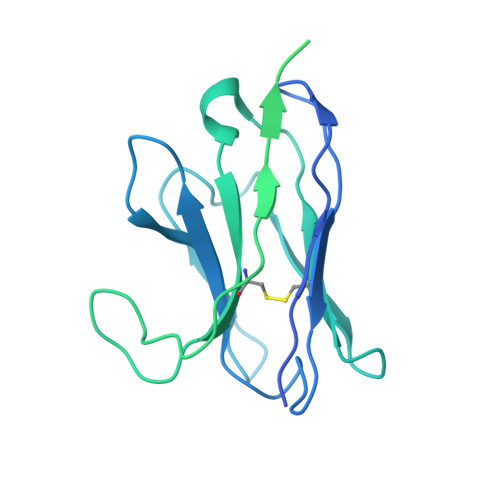
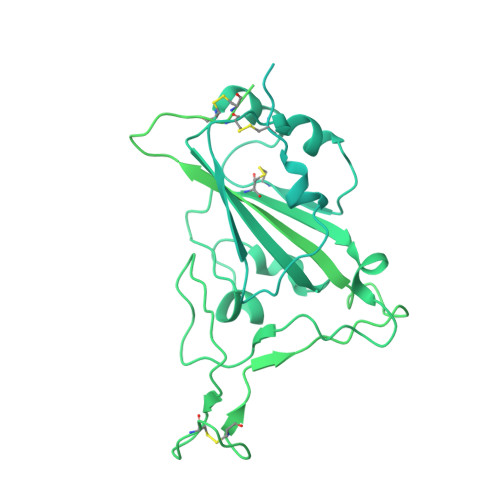
Avidity engineering of human heavy-chain-only antibodies mitigates neutralization resistance of SARS-CoV-2 variants.
Du, W., Janssens, R., Mykytyn, A.Z., Li, W., Drabek, D., van Haperen, R., Chatziandreou, M., Rissmann, M., van der Lee, J., van Dortmondt, M., Martin, I.S., van Kuppeveld, F.J.M., Hurdiss, D.L., Haagmans, B.L., Grosveld, F., Bosch, B.J.(2023) Front Immunol 14: 1111385-1111385
- PubMed: 36895554
- DOI: https://doi.org/10.3389/fimmu.2023.1111385
- Primary Citation of Related Structures:
8C8P - PubMed Abstract:
Emerging SARS-CoV-2 variants have accrued mutations within the spike protein rendering most therapeutic monoclonal antibodies against COVID-19 ineffective. Hence there is an unmet need for broad-spectrum mAb treatments for COVID-19 that are more resistant to antigenically drifted SARS-CoV-2 variants. Here we describe the design of a biparatopic heavy-chain-only antibody consisting of six antigen binding sites recognizing two distinct epitopes in the spike protein NTD and RBD. The hexavalent antibody showed potent neutralizing activity against SARS-CoV-2 and variants of concern, including the Omicron sub-lineages BA.1, BA.2, BA.4 and BA.5, whereas the parental components had lost Omicron neutralization potency. We demonstrate that the tethered design mitigates the substantial decrease in spike trimer affinity seen for escape mutations for the hexamer components. The hexavalent antibody protected against SARS-CoV-2 infection in a hamster model. This work provides a framework for designing therapeutic antibodies to overcome antibody neutralization escape of emerging SARS-CoV-2 variants.
- Virology Section, Infectious Diseases and Immunology Division, Department of Biomolecular Health Sciences, Faculty of Veterinary Medicine, Utrecht University, Utrecht, Netherlands.
Organizational Affiliation: