Impact of Hydrophobic Chains in Five-Coordinate Glucoconjugate Pt(II) Anticancer Agents.
Annunziata, A., Imbimbo, P., Cucciolito, M.E., Ferraro, G., Langellotti, V., Marano, A., Melchiorre, M., Tito, G., Trifuoggi, M., Monti, D.M., Merlino, A., Ruffo, F.(2023) Int J Mol Sci 24
- PubMed: 36768690
- DOI: https://doi.org/10.3390/ijms24032369
- Primary Citation of Related Structures:
8BOV, 8BOY - PubMed Abstract:
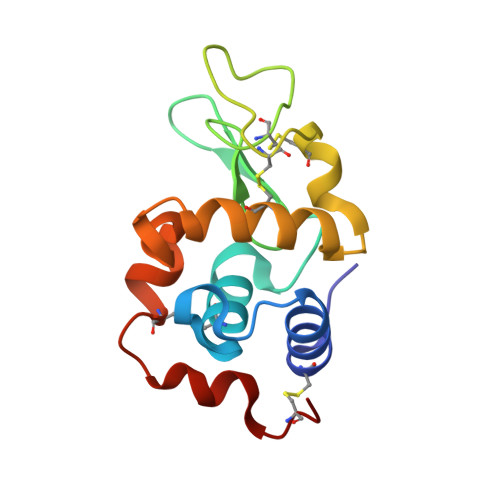
This study describes new platinum(II) cationic five-coordinate complexes ( 1-R,R' ) of the formula [PtR(NHC)(dmphen)(ethene)]CF 3 SO 3 (dmphen = 2,9-dimethyl-1,10-phenanthroline), containing in their axial positions an alkyl group R (methyl or octyl) and an imidazole-based NHC-carbene ligand with a substituent R' of variable length (methyl or octyl) on one nitrogen atom. The Pt-carbene bond is stable both in DMSO and in aqueous solvents. In DMSO, a gradual substitution of dmphen and ethene is observed, with the formation of a square planar solvated species. Octanol/water partitioning studies have revealed the order of hydrophobicity of the complexes ( 1-Oct,Me > 1-Oct,Oct > 1-Me,Oct > 1-Me,Me ). Their biological activity was investigated against two pairs of cancer and non-cancer cell lines. The tested drugs were internalized in cancer cells and able to activate the apoptotic pathway. The reactivity of 1-Me,Me with DNA and protein model systems was also studied using UV-vis absorption spectroscopy, fluorescence, and X-ray crystallography. The compound binds DNA and interacts in various ways with the model protein lysozyme. Remarkably, structural data revealed that the complex can bind lysozyme via non-covalent interactions, retaining its five-coordinate geometry.
- Institute Parisien de Chimie Moléculaire, Campus Pierre et Marie Curie, Sorbonne Université, 4 Place Jussieu, 75005 Paris, France.
Organizational Affiliation: