Biochemical and Structural Characterization of Chi-Class Glutathione Transferases: A Snapshot on the Glutathione Transferase Encoded by sll0067 Gene in the Cyanobacterium Synechocystis sp. Strain PCC 6803.
Mocchetti, E., Morette, L., Mulliert, G., Mathiot, S., Guillot, B., Dehez, F., Chauvat, F., Cassier-Chauvat, C., Brochier-Armanet, C., Didierjean, C., Hecker, A.(2022) Biomolecules 12
- PubMed: 36291676
- DOI: https://doi.org/10.3390/biom12101466
- Primary Citation of Related Structures:
8AI8, 8AI9, 8AIB - PubMed Abstract:
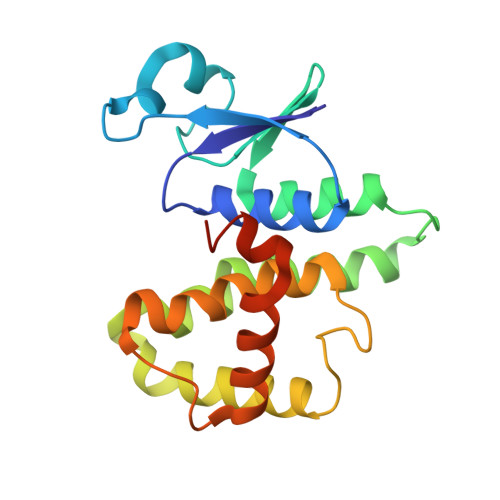
Glutathione transferases (GSTs) constitute a widespread superfamily of enzymes notably involved in detoxification processes and/or in specialized metabolism. In the cyanobacterium Synechocsytis sp. PCC 6803, SynGSTC1, a chi-class GST (GSTC), is thought to participate in the detoxification process of methylglyoxal, a toxic by-product of cellular metabolism. A comparative genomic analysis showed that GSTCs were present in all orders of cyanobacteria with the exception of the basal order Gloeobacterales. These enzymes were also detected in some marine and freshwater noncyanobacterial bacteria, probably as a result of horizontal gene transfer events. GSTCs were shorter of about 30 residues compared to most cytosolic GSTs and had a well-conserved SRAS motif in the active site ( 10 SRAS 13 in SynGSTC1). The crystal structure of SynGSTC1 in complex with glutathione adopted the canonical GST fold with a very open active site because the α4 and α5 helices were exceptionally short. A transferred multipolar electron-density analysis allowed a fine description of the solved structure. Unexpectedly, Ser10 did not have an electrostatic influence on glutathione as usually observed in serinyl-GSTs. The S10A variant was only slightly less efficient than the wild-type and molecular dynamics simulations suggested that S10 was a stabilizer of the protein backbone rather than an anchor site for glutathione.
- Université de Lorraine, CNRS, CRM2, F-54000 Nancy, France.
Organizational Affiliation: