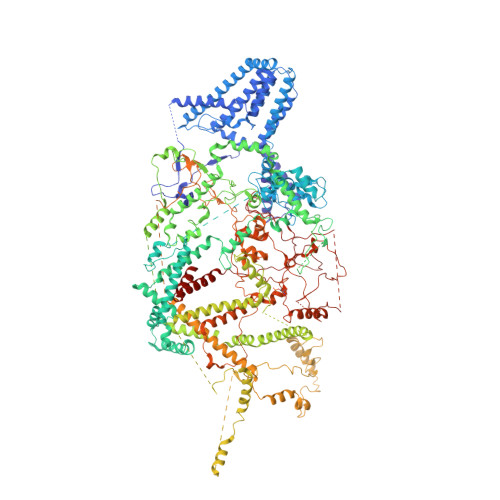
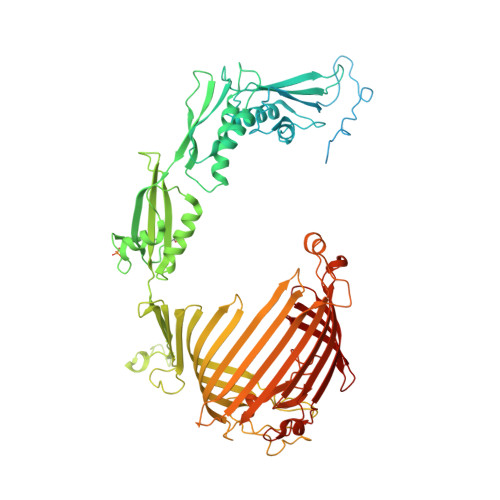
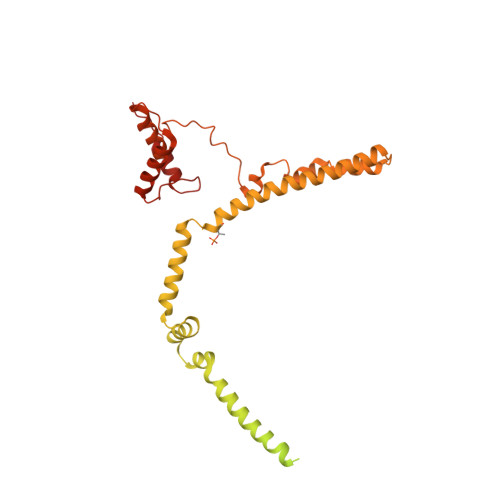
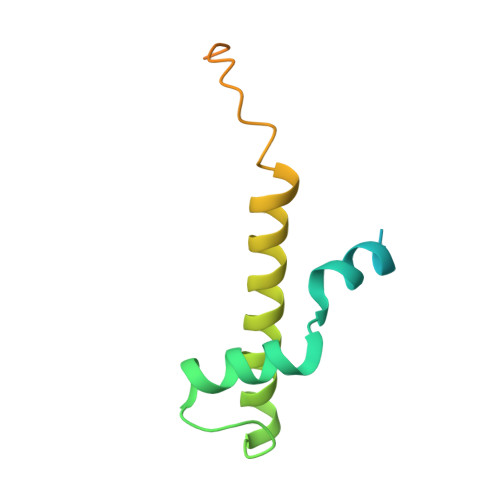
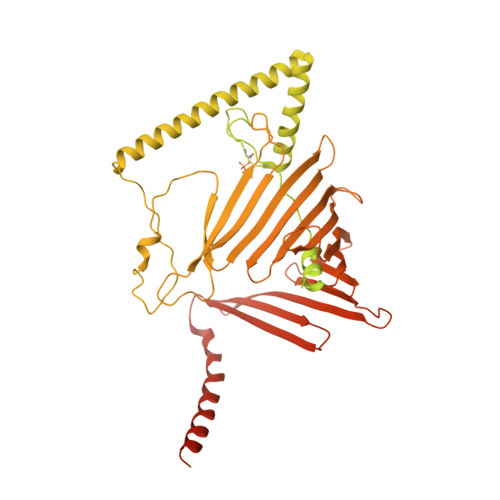
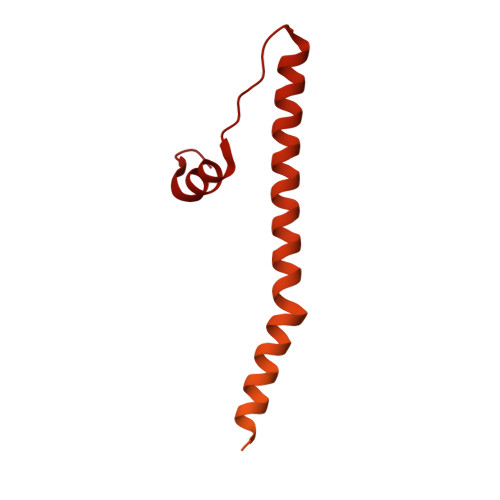
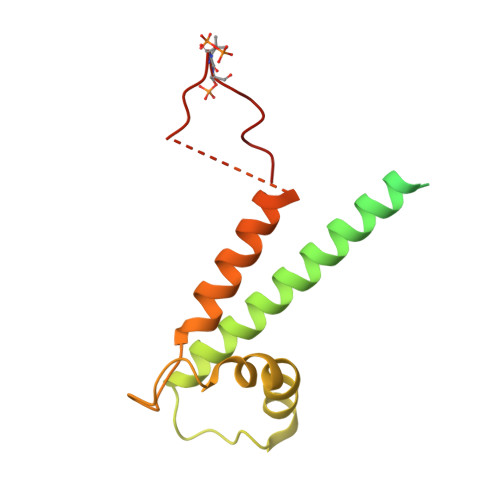
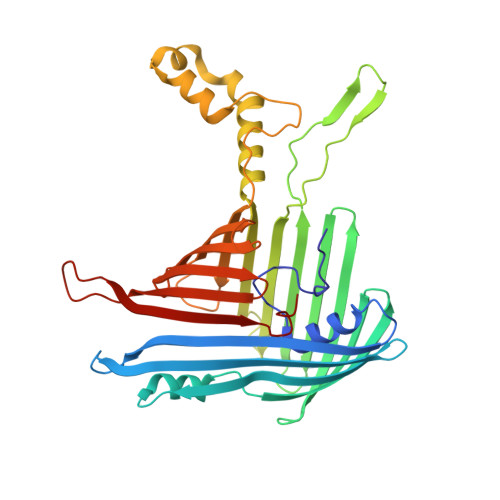
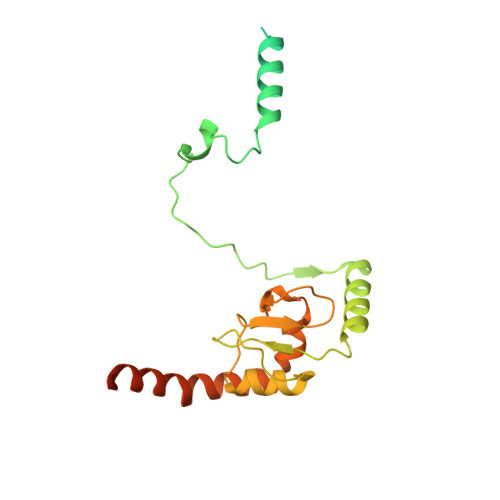
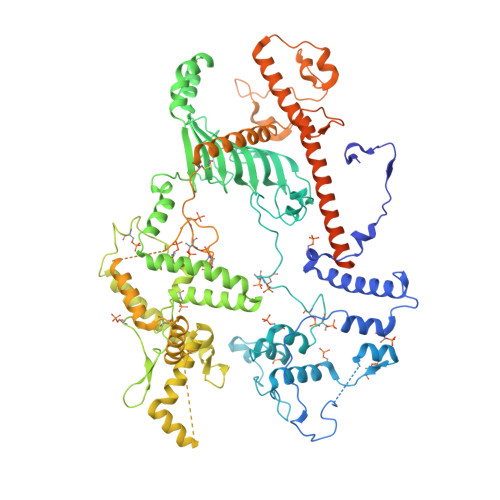
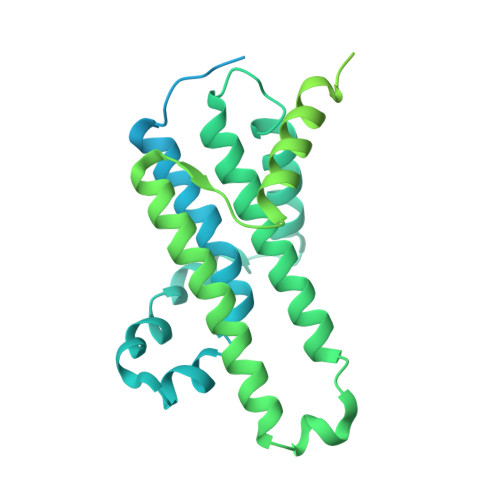
Structure of a TOC-TIC supercomplex spanning two chloroplast envelope membranes.
Jin, Z., Wan, L., Zhang, Y., Li, X., Cao, Y., Liu, H., Fan, S., Cao, D., Wang, Z., Li, X., Pan, J., Dong, M.Q., Wu, J., Yan, Z.(2022) Cell 185: 4788-4800.e13
- PubMed: 36413996
- DOI: https://doi.org/10.1016/j.cell.2022.10.030
- Primary Citation of Related Structures:
7VCF - PubMed Abstract:
The TOC and TIC complexes are essential translocons that facilitate the import of the nuclear genome-encoded preproteins across the two envelope membranes of chloroplast, but their exact molecular identities and assembly remain unclear. Here, we report a cryoelectron microscopy structure of TOC-TIC supercomplex from Chlamydomonas, containing a total of 14 identified components. The preprotein-conducting pore of TOC is a hybrid β-barrel co-assembled by Toc120 and Toc75, while the potential translocation path of TIC is formed by transmembrane helices from Tic20 and YlmG, rather than a classic model of Tic110. A rigid intermembrane space (IMS) scaffold bridges two chloroplast membranes, and a large hydrophilic cleft on the IMS scaffold connects TOC and TIC, forming a pathway for preprotein translocation. Our study provides structural insights into the TOC-TIC supercomplex composition, assembly, and preprotein translocation mechanism, and lays a foundation to interpret the evolutionary conservation and diversity of this fundamental translocon machinery.
- Key Laboratory of Structural Biology of Zhejiang Province, School of Life Sciences, Westlake University, Hangzhou, Zhejiang 310024, China; Westlake Laboratory of Life Sciences and Biomedicine, Hangzhou, Zhejiang 310024, China; Institute of Biology, Westlake Institute for Advanced Study, Hangzhou, Zhejiang 310024, China.
Organizational Affiliation: