Multimodal tubulin binding by the yeast kinesin-8, Kip3, underlies its motility and depolymerization
Arellano-Santoyo, H., Hernandez-Lopez, R.A., Stokasimov, E., Wang, R.Y.R., Pellman, D., Leschziner, A.E.(2021) bioRxiv
Experimental Data Snapshot
Starting Models: experimental
View more details
wwPDB Validation 3D Report Full Report
(2021) bioRxiv
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tubulin alpha-1A chain | 451 | Sus scrofa | Mutation(s): 0 EC: 3.6.5 | 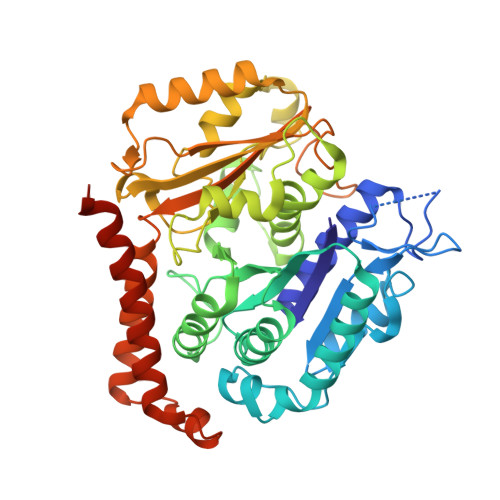 | |
UniProt | |||||
Find proteins for P02550 (Sus scrofa) Explore P02550 Go to UniProtKB: P02550 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P02550 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tubulin beta chain | 445 | Sus scrofa | Mutation(s): 0 | 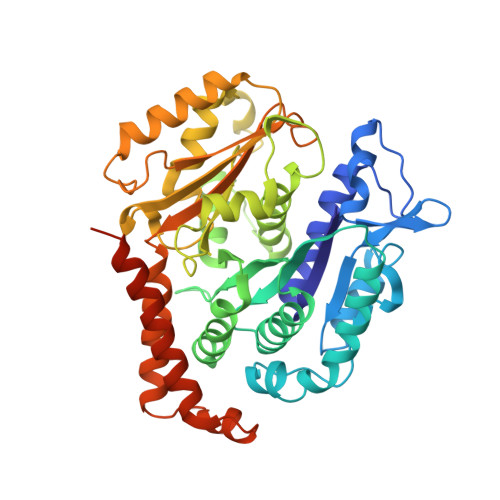 | |
UniProt | |||||
Find proteins for P02554 (Sus scrofa) Explore P02554 Go to UniProtKB: P02554 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P02554 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| yeast kinesin-8/ Kip3 | 355 | Saccharomyces cerevisiae | Mutation(s): 0 | 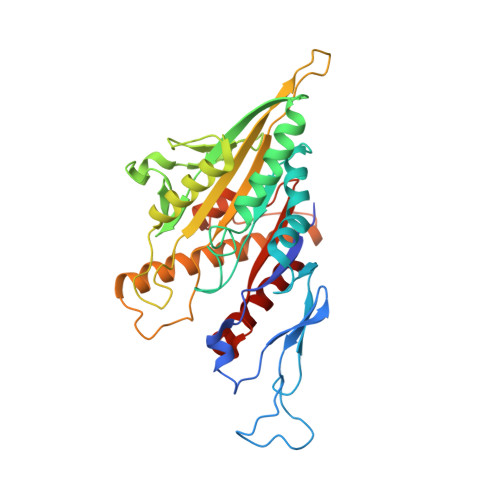 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| TA1 (Subject of Investigation/LOI) Query on TA1 | AC [auth S] CB [auth J] EA [auth B] IB [auth M] KA [auth D] | TAXOL C47 H51 N O14 RCINICONZNJXQF-MZXODVADSA-N |  | ||
| GTP (Subject of Investigation/LOI) Query on GTP | BA [auth A] FB [auth L] HA [auth C] LB [auth N] NA [auth E] | GUANOSINE-5'-TRIPHOSPHATE C10 H16 N5 O14 P3 XKMLYUALXHKNFT-UUOKFMHZSA-N |  | ||
| ANP (Subject of Investigation/LOI) Query on ANP | BC [auth h] DB [auth d] FA [auth K] JB [auth e] LA [auth a] | PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER C10 H17 N6 O12 P3 PVKSNHVPLWYQGJ-KQYNXXCUSA-N |  | ||
| GDP (Subject of Investigation/LOI) Query on GDP | BB [auth J] DA [auth B] HB [auth M] JA [auth D] NB [auth O] | GUANOSINE-5'-DIPHOSPHATE C10 H15 N5 O11 P2 QGWNDRXFNXRZMB-UUOKFMHZSA-N |  | ||
| MG (Subject of Investigation/LOI) Query on MG | AB [auth I] CA [auth A] CC [auth h] EB [auth d] GA [auth K] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | Rosetta | |
| RECONSTRUCTION | FREALIGN |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |