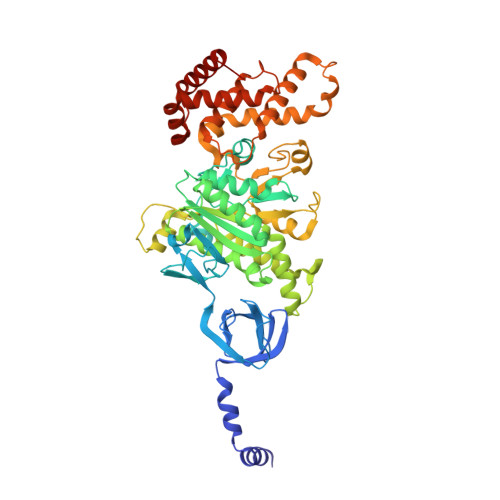
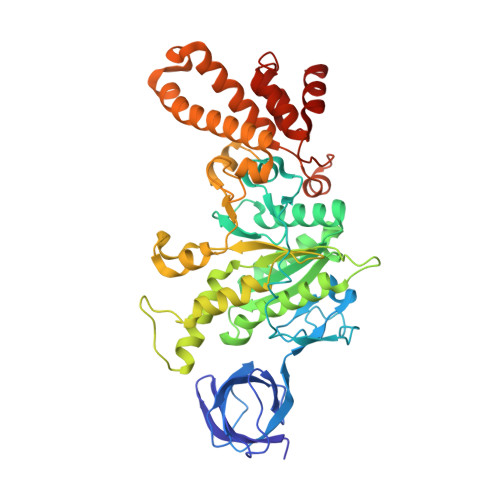
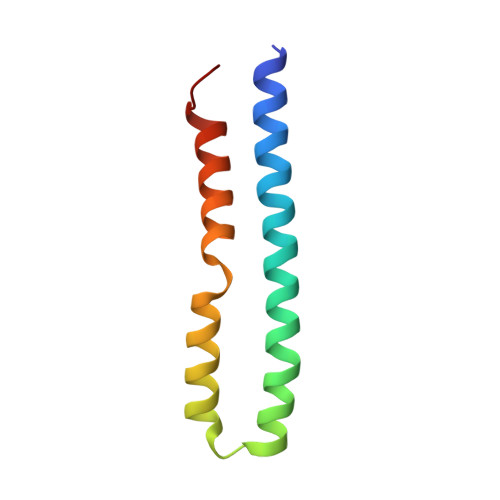
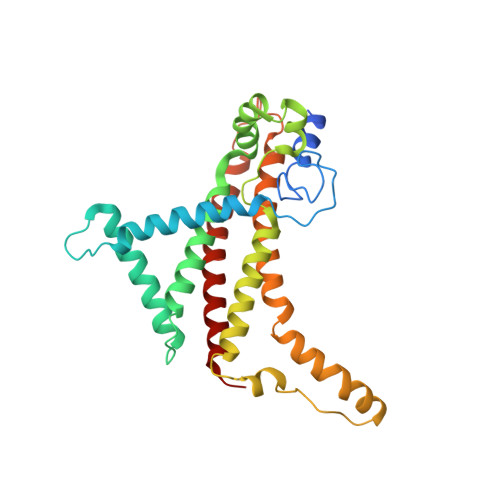
Structure of ATP synthase from ESKAPE pathogen Acinetobacter baumannii.
Demmer, J.K., Phillips, B.P., Uhrig, O.L., Filloux, A., Allsopp, L.P., Bublitz, M., Meier, T.(2022) Sci Adv 8: eabl5966-eabl5966
- PubMed: 35171679
- DOI: https://doi.org/10.1126/sciadv.abl5966
- Primary Citation of Related Structures:
7P2Y, 7P3N, 7P3W - PubMed Abstract:
The global spread of multidrug-resistant Acinetobacter baumannii infections urgently calls for the identification of novel drug targets. We solved the electron cryo-microscopy structure of the F 1 F o -adenosine 5'-triphosphate (ATP) synthase from A. baumannii in three distinct conformational states. The nucleotide-converting F 1 subcomplex reveals a specific self-inhibition mechanism, which supports a unidirectional ratchet mechanism to avoid wasteful ATP consumption. In the membrane-embedded F o complex, the structure shows unique structural adaptations along both the entry and exit pathways of the proton-conducting a-subunit. These features, absent in mitochondrial ATP synthases, represent attractive targets for the development of next-generation therapeutics that can act directly at the culmination of bioenergetics in this clinically relevant pathogen.
- Department of Life Sciences, Imperial College London, Exhibition Road, London SW7 2AZ, UK.
Organizational Affiliation: