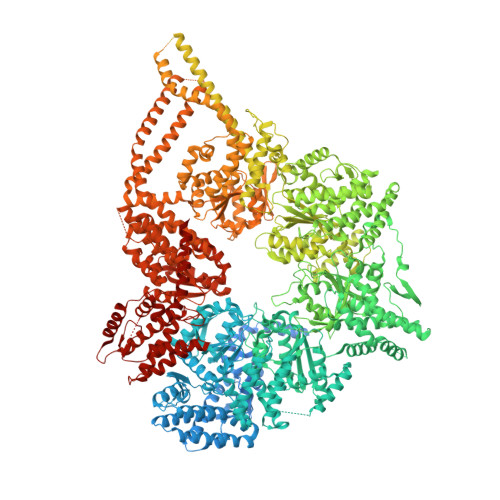
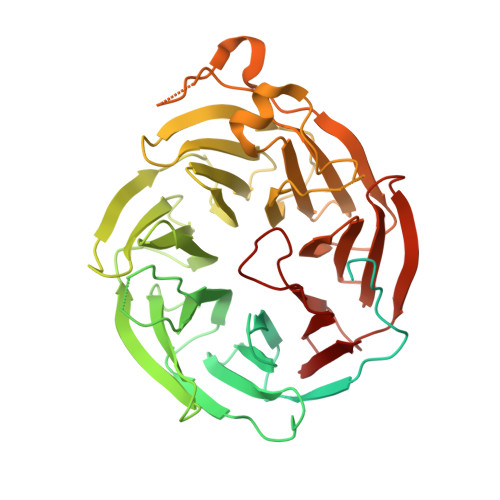
Structural basis for cytoplasmic dynein-1 regulation by Lis1.
Gillies, J.P., Reimer, J.M., Karasmanis, E.P., Lahiri, I., Htet, Z.M., Leschziner, A.E., Reck-Peterson, S.L.(2022) Elife 11
- PubMed: 34994688
- DOI: https://doi.org/10.7554/eLife.71229
- Primary Citation of Related Structures:
7MGM - PubMed Abstract:
The lissencephaly 1 gene, LIS1 , is mutated in patients with the neurodevelopmental disease lissencephaly. The Lis1 protein is conserved from fungi to mammals and is a key regulator of cytoplasmic dynein-1, the major minus-end-directed microtubule motor in many eukaryotes. Lis1 is the only dynein regulator known to bind directly to dynein's motor domain, and by doing so alters dynein's mechanochemistry. Lis1 is required for the formation of fully active dynein complexes, which also contain essential cofactors: dynactin and an activating adaptor. Here, we report the first high-resolution structure of the yeast dynein-Lis1 complex. Our 3.1 Å structure reveals, in molecular detail, the major contacts between dynein and Lis1 and between Lis1's ß-propellers. Structure-guided mutations in Lis1 and dynein show that these contacts are required for Lis1's ability to form fully active human dynein complexes and to regulate yeast dynein's mechanochemistry and in vivo function.
- Department of Cellular and Molecular Medicine, University of California, San Diego, San Diego, United States.
Organizational Affiliation: