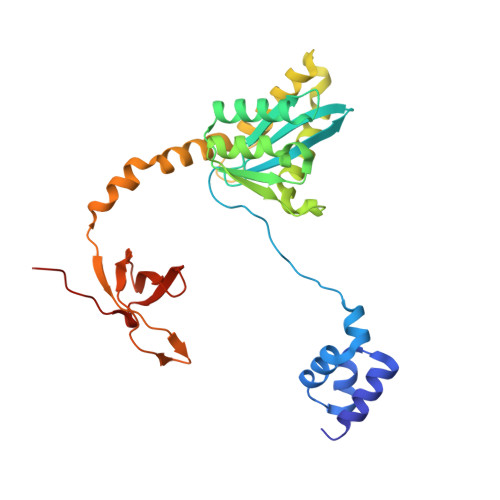
A supramolecular assembly mediates lentiviral DNA integration
Ballandras-Colas, A., Maskell, D.P., Serrao, E., Locke, J., Swuec, P., Jonsson, S.R., Kotecha, A., Cook, N.J., Pye, V.E., Taylor, I.A., Andresdottir, V., Engelman, A.N., Costa, A., Cherepanov, P.(2017) Science 355: 93-95
- PubMed: 28059770
- DOI: https://doi.org/10.1126/science.aah7002
- Primary Citation of Related Structures:
5LLJ, 5M0R, 5T3A, 7ZPP - PubMed Abstract:
Retroviral integrase (IN) functions within the intasome nucleoprotein complex to catalyze insertion of viral DNA into cellular chromatin. Using cryo-electron microscopy, we now visualize the functional maedi-visna lentivirus intasome at 4.9 angstrom resolution. The intasome comprises a homo-hexadecamer of IN with a tetramer-of-tetramers architecture featuring eight structurally distinct types of IN protomers supporting two catalytically competent subunits. The conserved intasomal core, previously observed in simpler retroviral systems, is formed between two IN tetramers, with a pair of C-terminal domains from flanking tetramers completing the synaptic interface. Our results explain how HIV-1 IN, which self-associates into higher-order multimers, can form a functional intasome, reconcile the bulk of early HIV-1 IN biochemical and structural data, and provide a lentiviral platform for design of HIV-1 IN inhibitors.
- Chromatin Structure and Mobile DNA, The Francis Crick Institute, London, NW1 1AT, UK.
Organizational Affiliation: