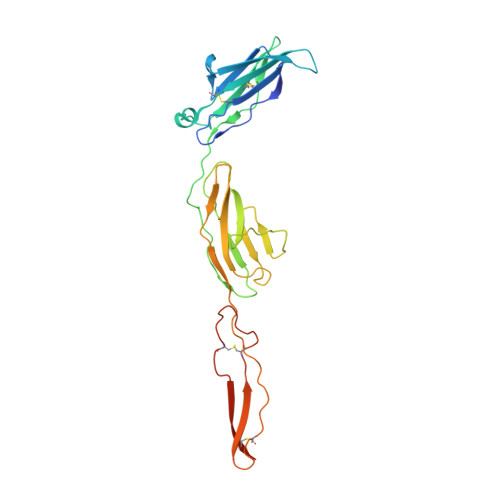
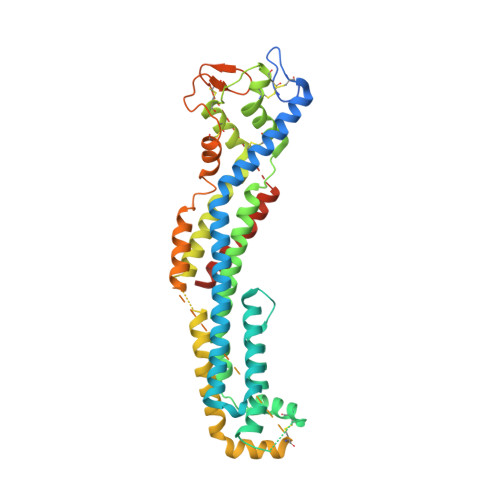
GPC3-Unc5 receptor complex structure and role in cell migration.
Akkermans, O., Delloye-Bourgeois, C., Peregrina, C., Carrasquero-Ordaz, M., Kokolaki, M., Berbeira-Santana, M., Chavent, M., Reynaud, F., Raj, R., Agirre, J., Aksu, M., White, E.S., Lowe, E., Ben Amar, D., Zaballa, S., Huo, J., Pakos, I., McCubbin, P.T.N., Comoletti, D., Owens, R.J., Robinson, C.V., Castellani, V., Del Toro, D., Seiradake, E.(2022) Cell 185: 3931-3949.e26
- PubMed: 36240740
- DOI: https://doi.org/10.1016/j.cell.2022.09.025
- Primary Citation of Related Structures:
7ZA1, 7ZA2, 7ZA3, 7ZAV, 7ZAW - PubMed Abstract:
Neural migration is a critical step during brain development that requires the interactions of cell-surface guidance receptors. Cancer cells often hijack these mechanisms to disseminate. Here, we reveal crystal structures of Uncoordinated-5 receptor D (Unc5D) in complex with morphogen receptor glypican-3 (GPC3), forming an octameric glycoprotein complex. In the complex, four Unc5D molecules pack into an antiparallel bundle, flanked by four GPC3 molecules. Central glycan-glycan interactions are formed by N-linked glycans emanating from GPC3 (N241 in human) and C-mannosylated tryptophans of the Unc5D thrombospondin-like domains. MD simulations, mass spectrometry and structure-based mutants validate the crystallographic data. Anti-GPC3 nanobodies enhance or weaken Unc5-GPC3 binding and, together with mutant proteins, show that Unc5/GPC3 guide migrating pyramidal neurons in the mouse cortex, and cancer cells in an embryonic xenograft neuroblastoma model. The results demonstrate a conserved structural mechanism of cell guidance, where finely balanced Unc5-GPC3 interactions regulate cell migration.
- Department of Biochemistry, University of Oxford, Oxford, UK.
Organizational Affiliation: