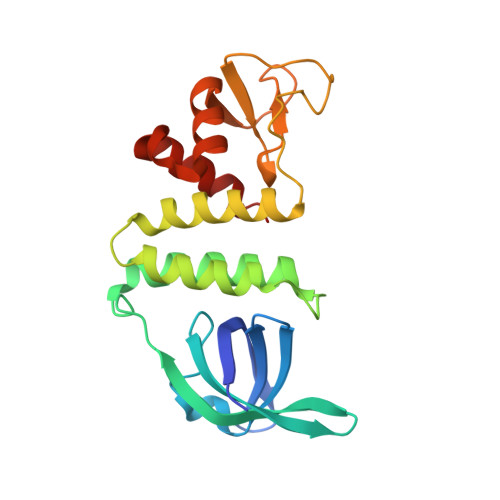
Crystal Structure of the Recombination Mediator Protein RecO from Campylobacter jejuni and Its Interaction with DNA and a Zinc Ion.
Lee, S.J., Oh, H.B., Yoon, S.I.(2022) Int J Mol Sci 23
- PubMed: 36077065
- DOI: https://doi.org/10.3390/ijms23179667
- Primary Citation of Related Structures:
7YMO - PubMed Abstract:
Homologous recombination is involved in repairing DNA damage, contributing to maintaining the integrity and stability of viral and cellular genomes. In bacteria, the recombination mediator proteins RecO and RecR are required to load the RecA recombinase on ssDNA for homologous recombination. To structurally and functionally characterize RecO, we determined the crystal structure of RecO from Campylobacter jejuni (cjRecO) at a 1.8 Å resolution and biochemically assessed its capacity to interact with DNA and a metal ion. cjRecO folds into a curved rod-like structure that consists of an N-terminal domain (NTD), C-terminal domain (CTD), and Zn 2+ -binding domain (ZnD). The ZnD at the end of the rod-like structure coordinates three cysteine residues and one histidine residue to accommodate a Zn 2+ ion. Based on an extensive comparative analysis of RecO structures and sequences, we propose that the Zn 2+ -binding consensus sequence of RecO is CxxC…C/HxxC/H/D. The interaction with Zn 2+ is indispensable for the protein stability of cjRecO but does not seem to be required for the recombination mediator function. cjRecO also interacts with ssDNA as part of its biological function, potentially using the positively charged patch in the NTD and CTD. However, cjRecO displays a low ssDNA-binding affinity, suggesting that cjRecO requires RecR to efficiently recognize ssDNA for homologous recombination.
- Division of Biomedical Convergence, College of Biomedical Science, Kangwon National University, Chuncheon 24341, Korea.
Organizational Affiliation: