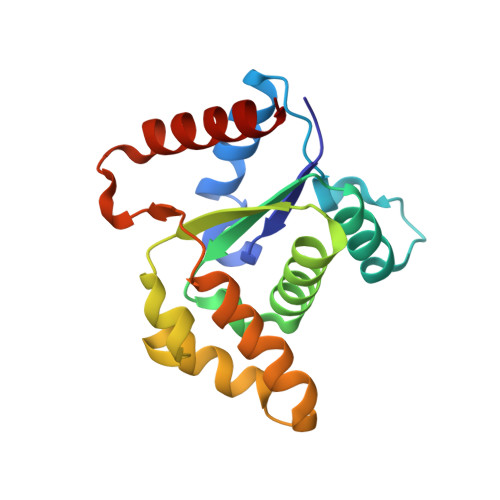
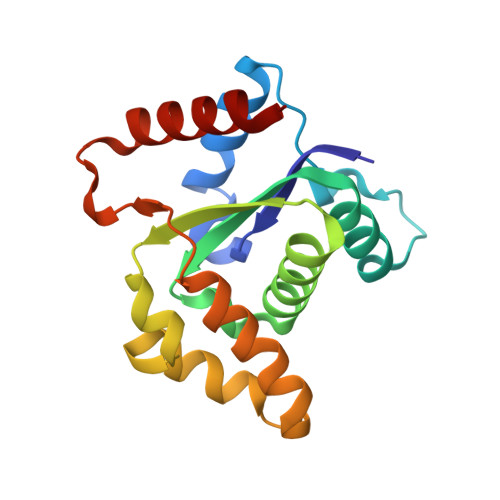
TIR-catalyzed ADP-ribosylation reactions produce signaling molecules for plant immunity.
Jia, A., Huang, S., Song, W., Wang, J., Meng, Y., Sun, Y., Xu, L., Laessle, H., Jirschitzka, J., Hou, J., Zhang, T., Yu, W., Hessler, G., Li, E., Ma, S., Yu, D., Gebauer, J., Baumann, U., Liu, X., Han, Z., Chang, J., Parker, J.E., Chai, J.(2022) Science 377: eabq8180-eabq8180
- PubMed: 35857644
- DOI: https://doi.org/10.1126/science.abq8180
- Primary Citation of Related Structures:
7XJP, 7XOZ - PubMed Abstract:
Plant pathogen-activated immune signaling by nucleotide-binding leucine-rich repeat (NLR) receptors with an N-terminal Toll/interleukin-1 receptor (TIR) domain converges on Enhanced Disease Susceptibility 1 (EDS1) and its direct partners, Phytoalexin Deficient 4 (PAD4) or Senescence-Associated Gene 101 (SAG101). TIR-encoded nicotinamide adenine dinucleotide hydrolase (NADase) produces signaling molecules to promote exclusive EDS1-PAD4 and EDS1-SAG101 interactions with helper NLR subclasses. In this work, we show that TIR-containing proteins catalyze adenosine diphosphate (ADP)-ribosylation of adenosine triphosphate (ATP) and ADP ribose (ADPR) through ADPR polymerase-like and NADase activity, forming ADP-ribosylated ATP (ADPr-ATP) and ADPr-ADPR (di-ADPR), respectively. Specific binding of ADPr-ATP or di-ADPR allosterically promotes EDS1-SAG101 interaction with helper NLR N requirement gene 1A (NRG1A) in vitro and in planta. Our data reveal an enzymatic activity of TIRs that enables specific activation of the EDS1-SAG101-NRG1 immunity branch.
- Beijing Advanced Innovation Center for Structural Biology, Tsinghua-Peking Joint Center for Life Sciences, Center for Plant Biology, School of Life Sciences, Tsinghua University, 100084 Beijing, China.
Organizational Affiliation: