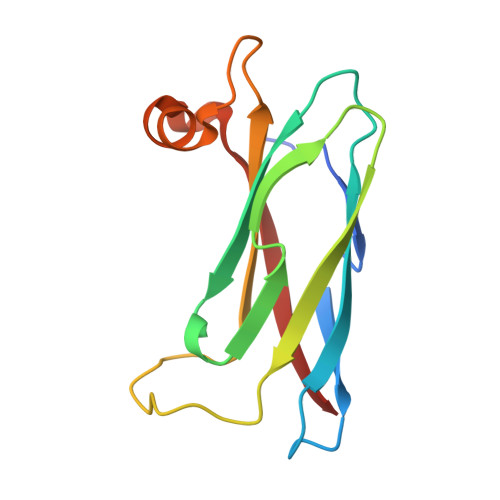
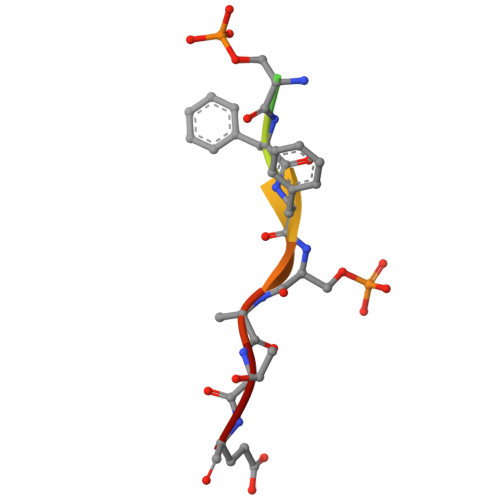
Structural basis for mitoguardin-2 mediated lipid transport at ER-mitochondrial membrane contact sites.
Kim, H., Lee, S., Jun, Y., Lee, C.(2022) Nat Commun 13: 3702-3702
- PubMed: 35764626
- DOI: https://doi.org/10.1038/s41467-022-31462-6
- Primary Citation of Related Structures:
7X14, 7X15 - PubMed Abstract:
The endoplasmic reticulum (ER)-mitochondria contact site (ERMCS) is crucial for exchanging biological molecules such as phospholipids and Ca 2+ ions between these organelles. Mitoguardin-2 (MIGA2), a mitochondrial outer membrane protein, forms the ERMCS in higher eukaryotic cells. Here, we report the crystal structures of the MIGA2 Lipid Droplet (LD) targeting domain and the ER membrane protein VAPB bound to the phosphorylated FFAT motif of MIGA2. These structures reveal that the MIGA2 LD targeting domain has a large internal hydrophobic pocket that accommodates phospholipids and that two phosphorylations of the FFAT motif are required for tight interaction of MIGA2 with VAPB, which enhances the rate of lipid transport. Further biochemical studies show that MIGA2 transports phospholipids between membranes with a strong preference for binding and trafficking phosphatidylserine (PS). These results provide a structural and molecular basis for understanding how MIGA2 mediates the formation of ERMCS and facilitates lipid trafficking at the ERMCS.
- Department of Biological Sciences, Ulsan National Institute of Science and Technology, Ulsan, Korea.
Organizational Affiliation: