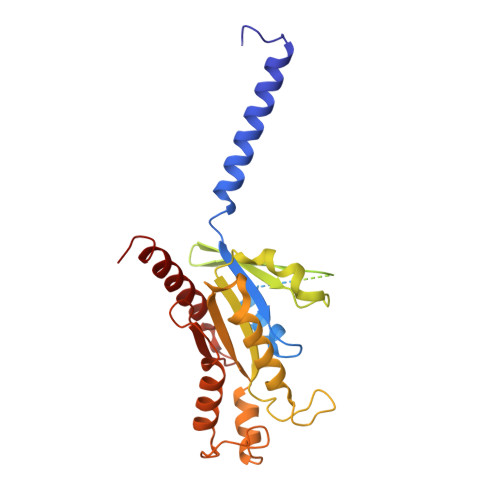
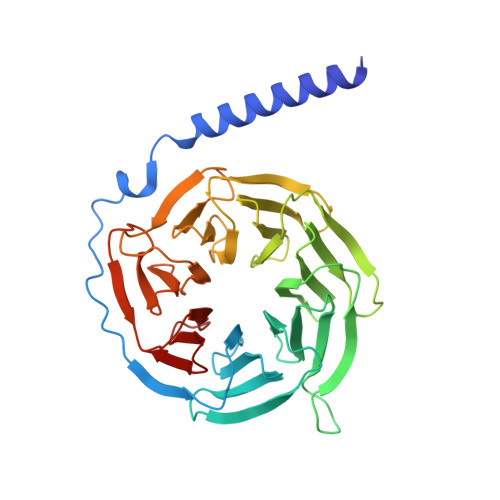
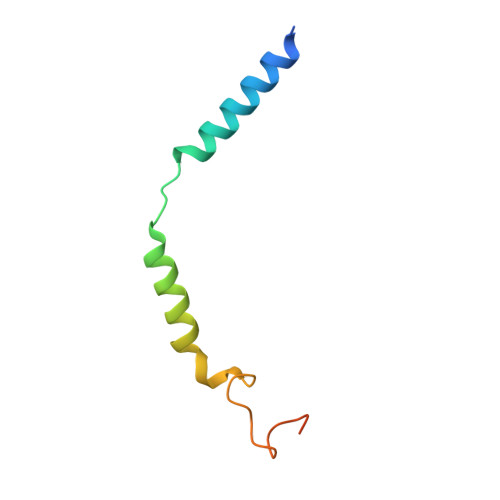
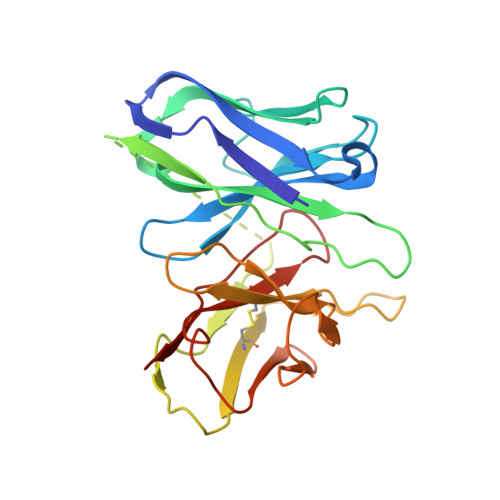
Structural insights into adhesion GPCR ADGRL3 activation and G q , G s , G i , and G 12 coupling.
Qian, Y., Ma, Z., Liu, C., Li, X., Zhu, X., Wang, N., Xu, Z., Xia, R., Liang, J., Duan, Y., Yin, H., Xiong, Y., Zhang, A., Guo, C., Chen, Z., Huang, Z., He, Y.(2022) Mol Cell 82: 4340-4352.e6
- PubMed: 36309016
- DOI: https://doi.org/10.1016/j.molcel.2022.10.009
- Primary Citation of Related Structures:
7WY5, 7WY8, 7WYB, 7X10 - PubMed Abstract:
Adhesion G-protein-coupled receptors (aGPCRs) play key roles in a diversity of physiologies. A hallmark of aGPCR activation is the removal of the inhibitory GAIN domain and the dipping of the cleaved stalk peptide into the ligand-binding pocket of receptors; however, the detailed mechanism remains obscure. Here, we present cryoelectron microscopy (cryo-EM) structures of ADGRL3 in complex with G q , G s , G i , and G 12 . The structures reveal unique ligand-engaging mode, distinctive activation conformation, and key mechanisms of aGPCR activation. The structures also reveal the uncharted structural information of GPCR/G 12 coupling. A comparison of G q , G s , G i , and G 12 engagements with ADGRL3 reveals the key determinant of G-protein coupling on the far end of αH5 of Gα. A detailed analysis of the engagements allows us to design mutations that specifically enhance one pathway over others. Taken together, our study lays the groundwork for understanding aGPCR activation and G-protein-coupling selectivity.
- Laboratory of Receptor Structure and Signaling, HIT Center for Life Sciences, Harbin Institute of Technology, Harbin 150001, China; HIT Center for Life Sciences, School of Life Science and Technology, Harbin Institute of Technology, Harbin 150080, China.
Organizational Affiliation: