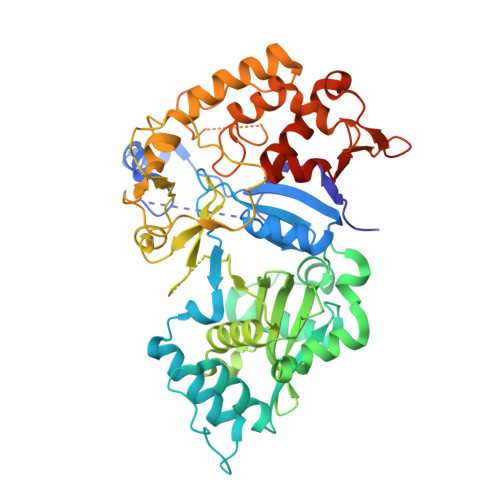
The Structure of Saccharomyces cerevisiae Arginyltransferase 1 (ATE1).
Van, V., Ejimogu, N.E., Bui, T.S., Smith, A.T.(2022) J Mol Biology 434: 167816-167816
- PubMed: 36087779
- DOI: https://doi.org/10.1016/j.jmb.2022.167816
- Primary Citation of Related Structures:
7TIF - PubMed Abstract:
Eukaryotic post-translational arginylation, mediated by the family of enzymes known as the arginyltransferases (ATE1s), is an important post-translational modification that can alter protein function and even dictate cellular protein half-life. Multiple major biological pathways are linked to the fidelity of this process, including neural and cardiovascular developments, cell division, and even the stress response. Despite this significance, the structural, mechanistic, and regulatory mechanisms that govern ATE1 function remain enigmatic. To that end, we have used X-ray crystallography to solve the crystal structure of ATE1 from the model organism Saccharomyces cerevisiae ATE1 (ScATE1) in the apo form. The three-dimensional structure of ScATE1 reveals a bilobed protein containing a GCN5-related N-acetyltransferase (GNAT) fold, and this crystalline behavior is faithfully recapitulated in solution based on size-exclusion chromatography-coupled small angle X-ray scattering (SEC-SAXS) analyses and cryo-EM 2D class averaging. Structural superpositions and electrostatic analyses point to this domain and its domain-domain interface as the location of catalytic activity and tRNA binding, and these comparisons strongly suggest a mechanism for post-translational arginylation. Additionally, our structure reveals that the N-terminal domain, which we have previously shown to bind a regulatory [Fe-S] cluster, is dynamic and disordered in the absence of metal bound in this location, hinting at the regulatory influence of this region. When taken together, these insights bring us closer to answering pressing questions regarding the molecular-level mechanism of eukaryotic post-translational arginylation.
- Department of Chemistry and Biochemistry, University of Maryland, Baltimore County, Baltimore, MD 21250, USA. Electronic address: https://twitter.com/VernaVan.
Organizational Affiliation: