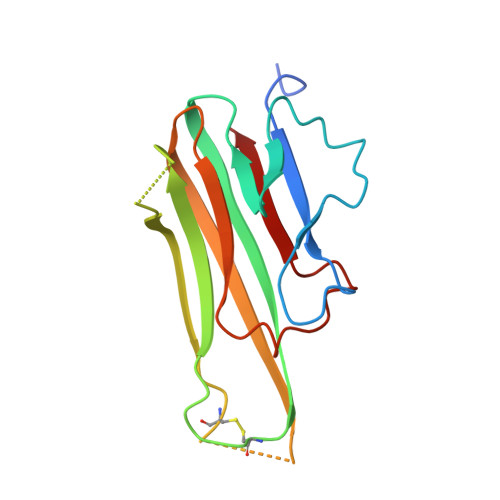
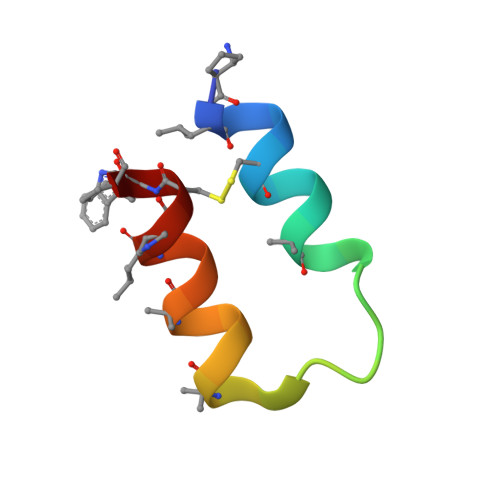
Trimer-to-Monomer Disruption Mechanism for a Potent, Protease-Resistant Antagonist of Tumor Necrosis Factor-alpha Signaling.
Niu, J., Cederstrand, A.J., Eddinger, G.A., Yin, B., Checco, J.W., Bingman, C.A., Outlaw, V.K., Gellman, S.H.(2022) J Am Chem Soc 144: 9610-9617
- PubMed: 35613436
- DOI: https://doi.org/10.1021/jacs.1c13717
- Primary Citation of Related Structures:
7TA3, 7TA6 - PubMed Abstract:
Aberrant tumor necrosis factor-α (TNFα) signaling is associated with many inflammatory diseases. The homotrimeric quaternary structure of TNFα is essential for receptor recognition and signal transduction. Previously, we described an engineered α/β-peptide inhibitor that potently suppresses TNFα activity and resists proteolysis. Here, we present structural evidence that both the α/β-peptide inhibitor and an all-α analogue bind to a monomeric form of TNFα. Calorimetry data support a 1:1 inhibitor/TNFα stoichiometry in solution. In contrast, previous cocrystal structures involving peptide or small-molecule inhibitors have shown the antagonists engaging a TNFα dimer. The structural data reveal why our inhibitors favor monomeric TNFα. Previous efforts to block TNFα-induced cell death with peptide inhibitors revealed that surfactant additives to the assay conditions cause a more rapid manifestation of inhibitory activity than is observed in the absence of additives. We attributed this effect to a loose surfactant TNFα association that lowers the barrier to trimer dissociation. Here, we used the new structural data to design peptide inhibitors bearing a surfactant-inspired appendage intended to facilitate TNFα trimer dissociation. The appendage modified the time course of protection from cell death.
- Department of Chemistry, University of Wisconsin-Madison, Madison, Wisconsin 53706, United States.
Organizational Affiliation: