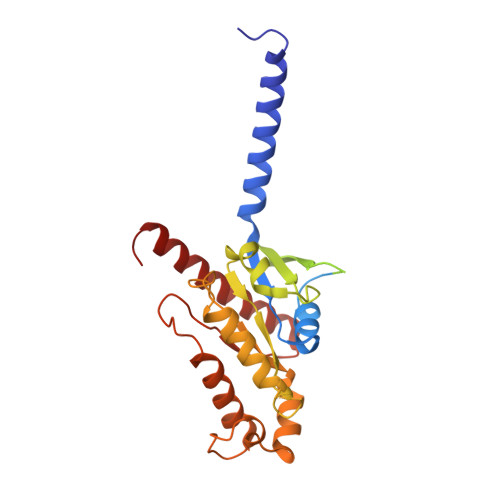
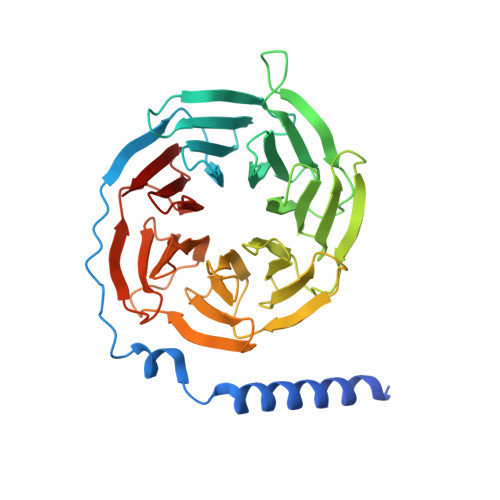
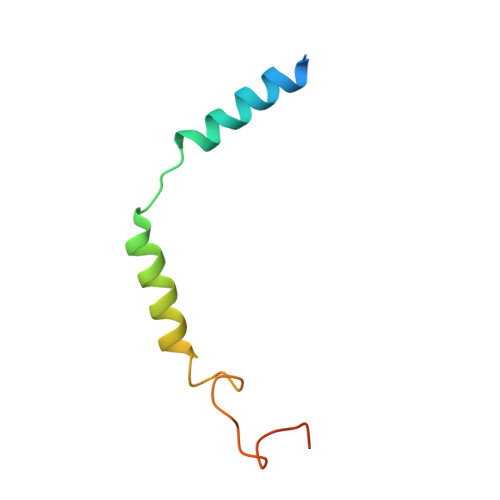
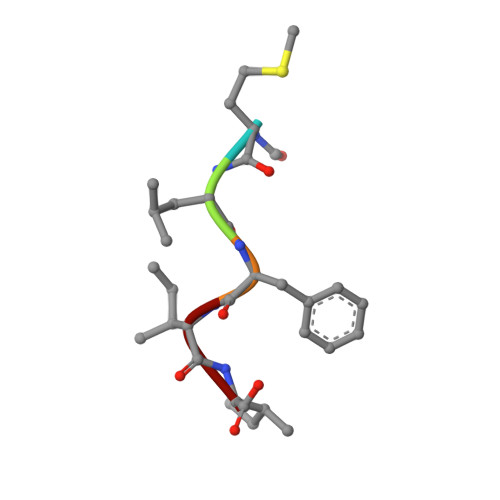
Molecular recognition of formylpeptides and diverse agonists by the formylpeptide receptors FPR1 and FPR2.
Zhuang, Y., Wang, L., Guo, J., Sun, D., Wang, Y., Liu, W., Xu, H.E., Zhang, C.(2022) Nat Commun 13: 1054-1054
- PubMed: 35217703
- DOI: https://doi.org/10.1038/s41467-022-28586-0
- Primary Citation of Related Structures:
7T6S, 7T6T, 7T6U, 7T6V - PubMed Abstract:
The formylpeptide receptors (FPRs) mediate pattern recognition of formylated peptides derived from invading pathogens or mitochondria from dead host cells. They can also sense other structurally distinct native peptides and even lipid mediators to either promote or resolve inflammation. Pharmacological targeting of FPRs represents a novel therapeutic approach in treating inflammatory diseases. However, the molecular mechanisms underlying FPR ligand recognition are elusive. We report cryo-EM structures of G i -coupled FPR1 and FPR2 bound to a formylpeptide and G i -coupled FPR2 bound to two synthetic peptide and small-molecule agonists. Together with mutagenesis data, our structures reveal the molecular mechanism of formylpeptide recognition by FPRs and structural variations of FPR1 and FPR2 leading to their different ligand preferences. Structural analysis also suggests that diverse FPR agonists sample a conserved activation chamber at the bottom of ligand-binding pockets to activate FPRs. Our results provide a basis for rational drug design on FPRs.
- The CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 201203, Shanghai, China.
Organizational Affiliation: