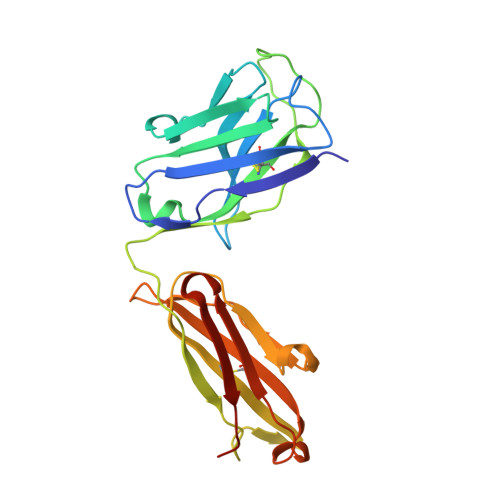
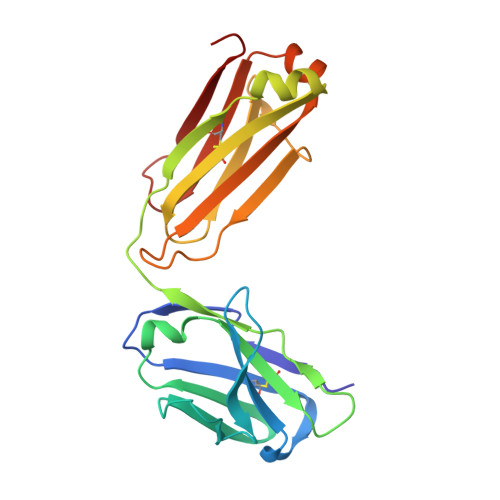
Epstein-Barr virus gH/gL has multiple sites of vulnerability for virus neutralization and fusion inhibition.
Chen, W.H., Kim, J., Bu, W., Board, N.L., Tsybovsky, Y., Wang, Y., Hostal, A., Andrews, S.F., Gillespie, R.A., Choe, M., Stephens, T., Yang, E.S., Pegu, A., Peterson, C.E., Fisher, B.E., Mascola, J.R., Pittaluga, S., McDermott, A.B., Kanekiyo, M., Joyce, M.G., Cohen, J.I.(2022) Immunity 55: 2135-2148.e6
- PubMed: 36306784
- DOI: https://doi.org/10.1016/j.immuni.2022.10.003
- Primary Citation of Related Structures:
7S07, 7S08, 7S0J, 7S1B - PubMed Abstract:
Epstein-Barr virus (EBV) is nearly ubiquitous in adults. EBV causes infectious mononucleosis and is associated with B cell lymphomas, epithelial cell malignancies, and multiple sclerosis. The EBV gH/gL glycoprotein complex facilitates fusion of virus membrane with host cells and is a target of neutralizing antibodies. Here, we examined the sites of vulnerability for virus neutralization and fusion inhibition within EBV gH/gL. We developed a panel of human monoclonal antibodies (mAbs) that targeted five distinct antigenic sites on EBV gH/gL and prevented infection of epithelial and B cells. Structural analyses using X-ray crystallography and electron microscopy revealed multiple sites of vulnerability and defined the antigenic landscape of EBV gH/gL. One mAb provided near-complete protection against viremia and lymphoma in a humanized mouse EBV challenge model. Our findings provide structural and antigenic knowledge of the viral fusion machinery, yield a potential therapeutic antibody to prevent EBV disease, and emphasize gH/gL as a target for herpesvirus vaccines and therapeutics.
- Emerging Infectious Diseases Branch, Walter Reed Army Institute of Research, Silver Spring, MD, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD 20817, USA.
Organizational Affiliation: