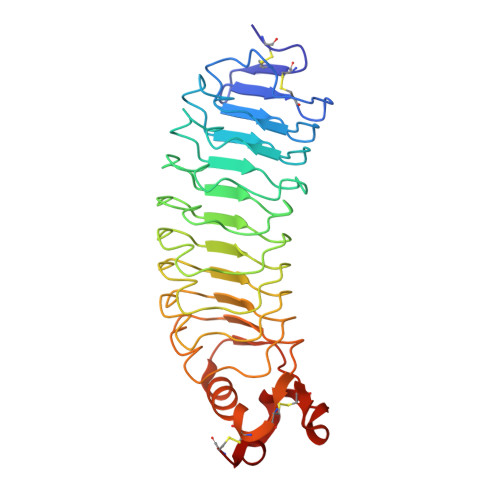
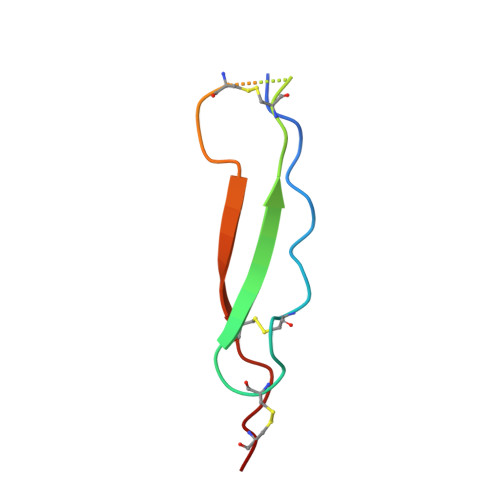
RTN4/NoGo-receptor binding to BAI adhesion-GPCRs regulates neuronal development.
Wang, J., Miao, Y., Wicklein, R., Sun, Z., Wang, J., Jude, K.M., Fernandes, R.A., Merrill, S.A., Wernig, M., Garcia, K.C., Sudhof, T.C.(2021) Cell 184: 5869-5885.e25
- PubMed: 34758294
- DOI: https://doi.org/10.1016/j.cell.2021.10.016
- Primary Citation of Related Structures:
7R84, 7R85, 7R86 - PubMed Abstract:
RTN4-binding proteins were widely studied as "NoGo" receptors, but their physiological interactors and roles remain elusive. Similarly, BAI adhesion-GPCRs were associated with numerous activities, but their ligands and functions remain unclear. Using unbiased approaches, we observed an unexpected convergence: RTN4 receptors are high-affinity ligands for BAI adhesion-GPCRs. A single thrombospondin type 1-repeat (TSR) domain of BAIs binds to the leucine-rich repeat domain of all three RTN4-receptor isoforms with nanomolar affinity. In the 1.65 Å crystal structure of the BAI1/RTN4-receptor complex, C-mannosylation of tryptophan and O-fucosylation of threonine in the BAI TSR-domains creates a RTN4-receptor/BAI interface shaped by unusual glycoconjugates that enables high-affinity interactions. In human neurons, RTN4 receptors regulate dendritic arborization, axonal elongation, and synapse formation by differential binding to glial versus neuronal BAIs, thereby controlling neural network activity. Thus, BAI binding to RTN4/NoGo receptors represents a receptor-ligand axis that, enabled by rare post-translational modifications, controls development of synaptic circuits.
- Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA 94305, USA.
Organizational Affiliation: